Remarkable Single Atom Catalyst of Transition Metal (Fe, Co & Ni) Doped on C2N Surface for Hydrogen Dissociation Reaction
- PMID: 36615939
- PMCID: PMC9823351
- DOI: 10.3390/nano13010029
Remarkable Single Atom Catalyst of Transition Metal (Fe, Co & Ni) Doped on C2N Surface for Hydrogen Dissociation Reaction
Abstract
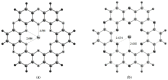
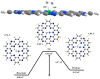
Currently, hydrogen is recognized as the best alternative for fossil fuels because of its sustainable nature and environmentally friendly processing. In this study, hydrogen dissociation reaction is studied theoretically on the transition metal doped carbon nitride (C2N) surface through single atom catalysis. Each TMs@C2N complex is evaluated to obtain the most stable spin state for catalytic reaction. In addition, electronic properties (natural bond orbital NBO & frontier molecular orbital FMO) of the most stable spin state complex are further explored. During dissociation, hydrogen is primarily adsorbed on metal doped C2N surface and then dissociated heterolytically between metal and nitrogen atom of C2N surface. Results revealed that theFe@C2N surface is the most suitable catalyst for H2 dissociation reaction with activation barrier of 0.36 eV compared with Ni@C2N (0.40 eV) and Co@C2N (0.45 eV) complexes. The activation barrier for H2 dissociation reaction is quite low in case of Fe@C2N surface, which is comparatively better than already reported noble metal catalysts.
Keywords: C2N surface; catalysis; hydrogen dissociation reaction; hydrogen energy; single atom catalyst; transition metals.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Figures






References
-
- Hagen J. Industrial Catalysis: A Practical Approach. John Wiley & Sons; Hoboken, NJ, USA: 2015.
-
- De Beer M., Kunene A., Nabaho D., Claeys M., Van Steen E. Technical and economic aspects of promotion of cobalt-based Fischer-Tropsch catalysts by noble metals—A review. J. South. Afr. Inst. Min. Metall. 2014;114:157–165.
Grants and funding
LinkOut - more resources
Full Text Sources

