Supramolecular Hydrogels from a Tripeptide and Carbon Nano-Onions for Biological Applications
- PMID: 36616081
- PMCID: PMC9824889
- DOI: 10.3390/nano13010172
Supramolecular Hydrogels from a Tripeptide and Carbon Nano-Onions for Biological Applications
Abstract
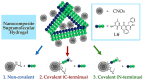
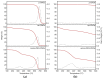
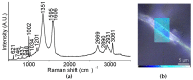
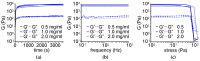
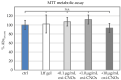
Nanocomposite hydrogels have attracted researchers' attention in recent years to achieve superior performances in a variety of materials applications. In this work, we describe the outcome of three different strategies to combine a self-assembling tripeptide and carbon nano-onions (CNOs), through covalent and non-covalent approaches, into supramolecular and nanostructured hydrogels. Importantly, the tripeptide coated the nano-onions and extended their aqueous dispersions' stability by several hours. Furthermore, CNOs could be loaded in the tripeptide hydrogels at the highest level ever reported for nanocarbons, indicating high compatibility between the components. The materials were formed in phosphate-buffered solutions, thus paving the way for biological applications, and were characterized by several spectroscopic, microscopic, thermogravimetric, and rheological techniques. In vitro experiments demonstrated excellent cytocompatibility.
Keywords: D-amino acids; biomaterials; carbon nano-onions; carbon nanomaterials; gels; hydrogelation; nanocomposites; peptides; self-assembly; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Muhammed Shameem M., Sasikanth S.M., Annamalai R., Ganapathi Raman R. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021;45:2536–2539. doi: 10.1016/j.matpr.2020.11.254. - DOI
-
- Melchionna M., Prato M. Functionalizing carbon nanotubes: An indispensible step towards applications. ECS J. Solid State Sci. Technol. 2013;2:M3040. doi: 10.1149/2.008310jss. - DOI
-
- Antonietti M., Bandosz T., Centi G., Costa R., Cruz-Silva R., Di J., Feng X., Frank B., Gebhardt P., Guldi D.M. Nanocarbon-Inorganic Hybrids: Next Generation Composites for Sustainable Energy Applications. Walter de Gruyter GmbH & Co., KG; Berlin, Germany: 2014.
Grants and funding
LinkOut - more resources
Full Text Sources

