Advances in the Mechanistic Understanding of Iron Oxide Nanoparticles' Radiosensitizing Properties
- PMID: 36616111
- PMCID: PMC9823929
- DOI: 10.3390/nano13010201
Advances in the Mechanistic Understanding of Iron Oxide Nanoparticles' Radiosensitizing Properties
Abstract
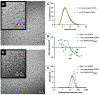
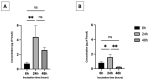
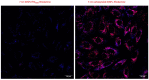
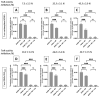
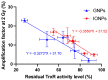
Among the plethora of nanosystems used in the field of theranostics, iron oxide nanoparticles (IONPs) occupy a central place because of their biocompatibility and magnetic properties. In this study, we highlight the radiosensitizing effect of two IONPs formulations (namely 7 nm carboxylated IONPs and PEG5000-IONPs) on A549 lung carcinoma cells when exposed to 225 kV X-rays after 6 h, 24 h and 48 h incubation. The hypothesis that nanoparticles exhibit their radiosensitizing effect by weakening cells through the inhibition of detoxification enzymes was evidenced by thioredoxin reductase activity monitoring. In particular, a good correlation between the amplification effect at 2 Gy and the residual activity of thioredoxin reductase was observed, which is consistent with previous observations made for gold nanoparticles (NPs). This emphasizes that NP-induced radiosensitization does not result solely from physical phenomena but also results from biological events.
Keywords: X-ray irradiation; biological mechanism; cancer therapy; iron oxide nanoparticles; radiosensitization; thioredoxin reductase.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment; consultancies; honoraria; stock ownership or options; expert testimony; grants or patents received or pending; or royalties.
Figures
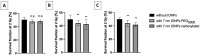
References
LinkOut - more resources
Full Text Sources
Miscellaneous

