Pyrolysis Kinetic Study of Polylactic Acid
- PMID: 36616361
- PMCID: PMC9823905
- DOI: 10.3390/polym15010012
Pyrolysis Kinetic Study of Polylactic Acid
Abstract
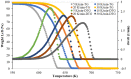
Polylactic acid (PLA) is a biodegradable polymer and is mainly used in the textile and food packaging fields. The aim of this work is to build knowledge on the kinetics of the pyrolysis of PLA with the help of thermogravimetric analysis (TGA) using four model-free methods, namely Friedman, Flynn-Wall-Qzawa (FWO), Kissinger-Akahira-Sunose (KAS), and Starink. Additionally, two model-fitting methods (the Coats-Redfern and Criado methods) were applied. TGA data at 5, 10, 20, and 30 K/min heating rates were collected. The obtained activation energies of the pyrolysis of PLA at different conversions by the model-free models were in good agreement and the average values were 97, 109, 104, and 104 kJ/mol for Friedman, FWO, KAS, and Starink, respectively. The Criado model was used together with the Coats-Redfern model to identify the most appropriate reaction mechanism. As per this work, the best controlling reaction mechanism of the PLA pyrolysis can be expressed by the geometrical contraction model (R2).
Keywords: PLA; activation energy; biodegradable polymer; kinetics; pyrolysis; recycling; thermogravimetric analyzer (TGA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Halász K., Csóka L. Plasticized biodegradable poly (lactic acid) based composites containing cellulose in micro-and nanosize. J. Eng. 2013;2013:329379. doi: 10.1155/2013/329379. - DOI
-
- Yuniarto K., Purwanto Y.A., Purwanto S., Welt B.A., Purwadaria H.K., Sunarti T.C. AIP Conference Proceedings. Volume 1725. AIP Publishing LLC; Melville, NY, USA: 2016. Infrared and Raman studies on polylactide acid and polyethylene glycol-400 blend; p. 020101. - DOI
-
- Dhar P., Tarafder D., Kumar A., Katiyar V. Effect of cellulose nanocrystal polymorphs on mechanical, barrier and thermal properties of poly (lactic acid) based bionanocomposites. RSC Adv. 2015;5:60426–60440. doi: 10.1039/C5RA06840A. - DOI
-
- Mortezaeikia V., Tavakoli O., Khodaparasti M.S. A review on kinetic study approach for pyrolysis of plastic wastes using thermogravimetric analysis. J. Anal. Appl. Pyrolysis. 2021;160:105340. doi: 10.1016/j.jaap.2021.105340. - DOI
-
- Saad J.M., Williams P.T., Zhang Y.S., Yao D., Yang H., Zhou H. Comparison of waste plastics pyrolysis under nitrogen and carbon dioxide atmospheres: A thermogravimetric and kinetic study. J. Anal. Appl. Pyrolysis. 2021;156:105135. doi: 10.1016/j.jaap.2021.105135. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

