Periodic Self-Assembly of Poly(ethyleneimine)-poly(4-styrenesulfonate) Complex Coacervate Membranes
- PMID: 36616395
- PMCID: PMC9824353
- DOI: 10.3390/polym15010045
Periodic Self-Assembly of Poly(ethyleneimine)-poly(4-styrenesulfonate) Complex Coacervate Membranes
Abstract
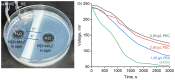
Coacervation is a self-assembly strategy based on the complexation of polyelectrolytes, which is utilized in biomedicine and agriculture, as well as automotive and textile industries. In this paper, we developed a new approach to the on-demand periodic formation of polyelectrolyte complexes through a Liesegang-type hierarchical organization. Adjustment of reaction conditions allows us to assemble materials with a tunable spatiotemporal geometry and establish materials' production cycles with a regulated periodicity. The proposed methodology allows the membrane to self-assemble when striving to reach balance and self-heal after exposure to external stimuli, such as potential difference and high pH. Using chronopotentiometry, K+ ion permeability behavior of the PEI-PSS coacervate membranes was demonstrated. The periodically self-assembled polyelectrolyte nanomembranes could further be integrated into novel energy storage devices and intelligent biocompatible membranes for bionics, soft nanorobotics, biosensing, and biocomputing.
Keywords: Liesegang rings; complex coacervation; ion permeability; periodic structure; polyelectrolytes; reaction-diffusion; self-healing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Skorb E.V., Andreeva D.V. Layer-by-Layer approaches for formation of smart self-healing materials. Polym. Chem. 2013;4:4834–4845. doi: 10.1039/c3py00088e. - DOI
-
- Campbell C.J., Baker E., Fialkowski M., Bitner A., Smoukov S.K., Grzybowski B.A. Self-organization of planar microlenses by periodic precipitation. J. Appl. Phys. 2005;97:126102. doi: 10.1063/1.1899757. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

