A Mini Review on the Development of Conjugated Polymers: Steps towards the Commercialization of Organic Solar Cells
- PMID: 36616512
- PMCID: PMC9853510
- DOI: 10.3390/polym15010164
A Mini Review on the Development of Conjugated Polymers: Steps towards the Commercialization of Organic Solar Cells
Abstract
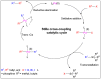
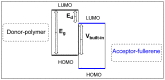
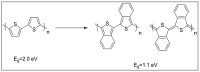
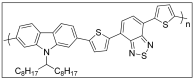
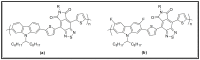
This review article covers the synthesis and design of conjugated polymers for carefully adjusting energy levels and energy band gap (EBG) to achieve the desired photovoltaic performance. The formation of bonds and the delocalization of electrons over conjugated chains are both explained by the molecular orbital theory (MOT). The intrinsic characteristics that classify conjugated polymers as semiconducting materials come from the EBG of organic molecules. A quinoid mesomeric structure (D-A ↔ D+ = A-) forms across the major backbones of the polymer as a result of alternating donor-acceptor segments contributing to the pull-push driving force between neighboring units, resulting in a smaller optical EBG. Furthermore, one of the most crucial factors in achieving excellent performance of the polymer is improving the morphology of the active layer. In order to improve exciton diffusion, dissociation, and charge transport, the nanoscale morphology ensures nanometer phase separation between donor and acceptor components in the active layer. It was demonstrated that because of the exciton's short lifetime, only small diffusion distances (10-20 nm) are needed for all photo-generated excitons to reach the interfacial region where they can separate into free charge carriers. There is a comprehensive explanation of the architecture of organic solar cells using single layer, bilayer, and bulk heterojunction (BHJ) devices. The short circuit current density (Jsc), open circuit voltage (Voc), and fill factor (FF) all have a significant impact on the performance of organic solar cells (OSCs). Since the BHJ concept was first proposed, significant advancement and quick configuration development of these devices have been accomplished. Due to their ability to combine great optical and electronic properties with strong thermal and chemical stability, conjugated polymers are unique semiconducting materials that are used in a wide range of applications. According to the fundamental operating theories of OSCs, unlike inorganic semiconductors such as silicon solar cells, organic photovoltaic devices are unable to produce free carrier charges (holes and electrons). To overcome the Coulombic attraction and separate the excitons into free charges in the interfacial region, organic semiconductors require an additional thermodynamic driving force. From the molecular engineering of conjugated polymers, it was discovered that the most crucial obstacles to achieving the most desirable properties are the design and synthesis of conjugated polymers toward optimal p-type materials. Along with plastic solar cells (PSCs), these materials have extended to a number of different applications such as light-emitting diodes (LEDs) and field-effect transistors (FETs). Additionally, the topics of fluorene and carbazole as donor units in conjugated polymers are covered. The Stille, Suzuki, and Sonogashira coupling reactions widely used to synthesize alternating D-A copolymers are also presented. Moreover, conjugated polymers based on anthracene that can be used in solar cells are covered.
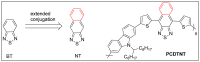
Keywords: Sonogashira coupling reaction; Suzuki coupling reaction; benzothiadiazole (BT); bulk heterojunction (BHJ) device; conjugated polymers; naphthothiadiazole (NT); organic solar cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



























Similar articles
-
Interfacial and Bulk Nanostructures Control Loss of Charges in Organic Solar Cells.Acc Chem Res. 2019 Oct 15;52(10):2904-2915. doi: 10.1021/acs.accounts.9b00331. Epub 2019 Oct 2. Acc Chem Res. 2019. PMID: 31577121
-
A new class of semiconducting polymers for bulk heterojunction solar cells with exceptionally high performance.Acc Chem Res. 2010 Sep 21;43(9):1227-36. doi: 10.1021/ar1000296. Acc Chem Res. 2010. PMID: 20853907
-
Molecular bulk heterojunctions: an emerging approach to organic solar cells.Acc Chem Res. 2009 Nov 17;42(11):1719-30. doi: 10.1021/ar900041b. Acc Chem Res. 2009. PMID: 19580313
-
Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review.Polymers (Basel). 2020 Nov 9;12(11):2627. doi: 10.3390/polym12112627. Polymers (Basel). 2020. PMID: 33182241 Free PMC article. Review.
-
π-Conjugated Polymers and Their Application in Organic and Hybrid Organic-Silicon Solar Cells.Polymers (Basel). 2022 Feb 13;14(4):716. doi: 10.3390/polym14040716. Polymers (Basel). 2022. PMID: 35215629 Free PMC article. Review.
Cited by
-
Building Block Engineering toward Realizing High-Performance Electrochromic Materials and Glucose Biosensing Platform.Biosensors (Basel). 2023 Jun 25;13(7):677. doi: 10.3390/bios13070677. Biosensors (Basel). 2023. PMID: 37504076 Free PMC article.
-
Glassy Fluorene and Sulfone Polyesters with High Refractive Index and Thermal Stability Whose Monomers Induce Estrogenic Bioactivity.ACS Omega. 2025 Feb 28;10(9):9613-9622. doi: 10.1021/acsomega.4c10782. eCollection 2025 Mar 11. ACS Omega. 2025. PMID: 40092783 Free PMC article.
-
Comparative Investigation of Ultrafast Excited-State Electron Transfer in Both Polyfluorene-Graphene Carboxylate and Polyfluorene-DCB Interfaces.Molecules. 2024 Jan 29;29(3):634. doi: 10.3390/molecules29030634. Molecules. 2024. PMID: 38338379 Free PMC article.
-
Nanomedicine in Neuroprotection, Neuroregeneration, and Blood-Brain Barrier Modulation: A Narrative Review.Medicina (Kaunas). 2024 Aug 24;60(9):1384. doi: 10.3390/medicina60091384. Medicina (Kaunas). 2024. PMID: 39336425 Free PMC article. Review.
-
Development of Benzobisoxazole-Based Novel Conjugated Polymers for Organic Thin-Film Transistors.Polymers (Basel). 2023 Feb 24;15(5):1156. doi: 10.3390/polym15051156. Polymers (Basel). 2023. PMID: 36904397 Free PMC article.
References
-
- Bloor D., Movaghar B. Conducting polymers. Solid-State Electron Devices IEE Proc. I. 1983;130:225–232. doi: 10.1049/ip-i-1.1983.0041. - DOI
-
- Chiang C.K., Gau S.C., Fincher C.R., Jr., Park Y.W., MacDiarmid A.G., Heeger A.J. Polyacetylene,(CH)x: n-type and p-type doping and compensation. Appl. Phys. Lett. 1978;33:18–20. doi: 10.1063/1.90166. - DOI
-
- Wegner G. Polymers with Metal-Like Conductivity—A Review of their Synthesis, Structure and Properties. Angew. Chem. Int. Ed. Engl. 1981;20:361–381. doi: 10.1002/anie.198103611. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

