On the bioprotective effects of 3-hydroxybutyrate: Thermodynamic study of binary 3HB-water systems
- PMID: 36617191
- PMCID: PMC9941717
- DOI: 10.1016/j.bpj.2023.01.004
On the bioprotective effects of 3-hydroxybutyrate: Thermodynamic study of binary 3HB-water systems
Abstract
Microorganisms must face various inconvenient conditions; therefore, they developed several approaches for protection. Such a strategy also involves the accumulation of compatible solutes, also called osmolytes. It has been proved that the monomer unit 3-hydroxybutyrate (3HB), which is present in sufficient concentration in poly(3-hydroxybutyrate) (PHB)-accumulating cells, serves as a chemical chaperone protecting enzymes against heat and oxidative stress and as a cryoprotectant for enzymes, bacterial cells, and yeast. The stress robustness of the cells is also strongly dependent on the behavior and state of intracellular water, especially during stress exposure. For a better understanding of the protective mechanism and effect of strongly hydrophilic 3HB in solutions at a wide range of temperatures, a binary phase diagram of system sodium 3HB (Na3HB)-water in equilibrium and the state diagrams showing the glass transitions in the system were constructed. To investigate the activity of water in various compositions of the Na3HB/water system, three experimental techniques have been used (dynamic water sorption analysis, water activity measurements, and sorption calorimetry). First, Na3HB proved its hydrophilic nature, which is very comparable with known compatible solutes (trehalose). Results of differential scanning calorimetry demonstrated that Na3HB is also highly effective in depressing the freezing point and generating a large amount of nonfrozen water (1.35 g of water per gram of Na3HB). Therefore, Na3HB represents a very effective cryoprotectant that can be widely used for numerous applications.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

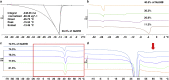

 ) and DVS (
) and DVS ( ). All lines are drawn as a guide for the eye to follow the respective phase boundaries.
). All lines are drawn as a guide for the eye to follow the respective phase boundaries.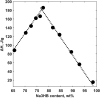

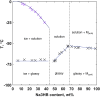

References
-
- Da Costa M.S., Santos H., Galinski E.A. In: Biotechnology of Extremophiles. Antranikian G., editor. Springer/Berlin; 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea; pp. 117–153. - PubMed
-
- Fuller B.J. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Lett. 2004;25:375–388. - PubMed
-
- Göller K., A Galinski E. Protection of a model enzyme lactate dehydrogenase against heat, urea and freeze-thaw treatment by compatible solute additives. J. Mol. Catal. B. Enzym. 1999;7:37–45. doi: 10.1016/S1381-11779900043-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

