Development of a Bispecific Antibody Targeting Clinical Isolates of Acinetobacter baumannii
- PMID: 36617220
- PMCID: PMC10319980
- DOI: 10.1093/infdis/jiac499
Development of a Bispecific Antibody Targeting Clinical Isolates of Acinetobacter baumannii
Abstract
Background: We previously reported developing 2 anticapsular monoclonal antibodies (mAbs) as a novel therapy for Acinetobacter baumannii infections. We sought to determine whether a bispecific mAb (bsAb) could improve avidity and efficacy while maximizing strain coverage in one molecule.
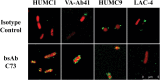
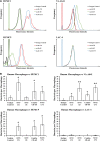
Methods: Humanized mAb 65 was cloned into a single-chain variable fragment and attached to humanized mAb C8, combining their paratopes into a single bsAb (C73). We tested bsAb C73's strain coverage, binding affinity, ex vivo opsonic activity, and in vivo efficacy compared to each mAb alone and combined.
Results: The bsAb demonstrated strain coverage, binding affinity, opsonization, and in vivo efficacy superior to either original mAb alone or combined.
Conclusions: A humanized bsAb targeting distinct A. baumannii capsule moieties enabled potent and effective coverage of disparate A. baumannii clinical isolates. The bsAb enhances feasibility of development by minimizing the number of components of a promising novel therapeutic for these difficult-to-treat infections.
Keywords: Acinetobacter baumannii; bispecific monoclonal antibody; carbapenem resistant; extreme drug resistance; immunotherapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . T. B. N. and B. S. are inventors on patents related to monoclonal antibodies C8 and 65. T. B. N., J. Y., B. M. L., and B. S. are inventors on a patent related to bispecific monoclonal antibody C73. T. B. N. and B. S. own equity in BioAIM, which is developing the antibodies. U. S. C. has an ownership interest in BioAIM and is entitled to a share of royalties based on a licensing agreement with BioAIM. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–301. - PMC - PubMed
-
- Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. - PubMed
-
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695–703. - PubMed

