Dabrafenib plus trametinib treatment in patients with anaplastic thyroid carcinoma: an Argentinian experience
- PMID: 36617605
- PMCID: PMC9838471
- DOI: 10.1007/s12020-022-03295-2
Dabrafenib plus trametinib treatment in patients with anaplastic thyroid carcinoma: an Argentinian experience
Abstract
Purpose: To present our real-life experience with dabrafenib and trametinib (D-T) treatment in patients with BRAF V600E-mutated ATC in Argentina.
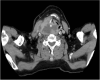
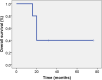
Patients y methods: We included five patients from four different hospitals. The median age was 70 years, and 60% were male. The performance status at diagnosis was grade 0 in 60% and grade 2 in 40% of patients. Four patients could undergo total thyroidectomy; in one of them, surgical treatment was amenable due to the indication of D-T as neoadjuvant therapy. From the total cohort, the best response to treatment was complete response in 40%, partial response in 20%, and stable disease in 20%. The median duration of response was 20 weeks, ranging from 16 to 92 weeks. All patients experienced at least one adverse event (AE). Grade ≥3 AEs were observed in two (40%) patients. They were upper gastrointestinal bleeding and subclavian vein thrombosis. The median follow-up was 20 weeks (range: 16 to 92).
Conclusion: This report contributes to illustrate the feasibility and effectiveness of D-T treatment in five patients with loco-regionally advanced and metastatic BRAF V600E-mutated ATC in a real-life setting. A multidisciplinary approach and rapid molecular-tailored testing are essential to begin this therapeutic option.
Keywords: Anaplastic thyroid carcinoma; Dabrafenib; Real-life experience; Targeted therapy; Trametinib.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

