FOXO3A-short is a novel regulator of non-oxidative glucose metabolism associated with human longevity
- PMID: 36617632
- PMCID: PMC10014046
- DOI: 10.1111/acel.13763
FOXO3A-short is a novel regulator of non-oxidative glucose metabolism associated with human longevity
Abstract
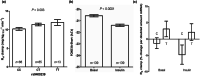
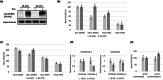
Intronic single-nucleotide polymorphisms (SNPs) in FOXO3A are associated with human longevity. Currently, it is unclear how these SNPs alter FOXO3A functionality and human physiology, thereby influencing lifespan. Here, we identify a primate-specific FOXO3A transcriptional isoform, FOXO3A-Short (FOXO3A-S), encoding a major longevity-associated SNP, rs9400239 (C or T), within its 5' untranslated region. The FOXO3A-S mRNA is highly expressed in the skeletal muscle and has very limited expression in other tissues. We find that the rs9400239 variant influences the stability and functionality of the primarily nuclear protein(s) encoded by the FOXO3A-S mRNA. Assessment of the relationship between the FOXO3A-S polymorphism and peripheral glucose clearance during insulin infusion (Rd clamp) in a cohort of Danish twins revealed that longevity T-allele carriers have markedly faster peripheral glucose clearance rates than normal lifespan C-allele carriers. In vitro experiments in human myotube cultures utilizing overexpression of each allele showed that the C-allele represses glycolysis independently of PI3K signaling, while overexpression of the T-allele represses glycolysis only in a PI3K-inactive background. Supporting this finding inducible knockdown of the FOXO3A-S C-allele in cultured myotubes increases the glycolytic rate. We conclude that the rs9400239 polymorphism acts as a molecular switch which changes the identity of the FOXO3A-S-derived protein(s), which in turn alters the relationship between FOXO3A-S and insulin/PI3K signaling and glycolytic flux in the skeletal muscle. This critical difference endows carriers of the FOXO3A-S T-allele with consistently higher insulin-stimulated peripheral glucose clearance rates, which may contribute to their longer and healthier lifespans.
Keywords: FOXO; FOXO3A; PI3K; SNP; aging; glycolysis; insulin; skeletal muscle.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Arum, O. , Boparai, R. K. , Saleh, J. K. , Wang, F. , Dirks, A. L. , Turner, J. G. , Kopchick, J. J. , Liu, J. L. , Khardori, R. K. , & Bartke, A. (2014). Specific suppression of insulin sensitivity in growth hormone receptor gene‐disrupted (GHR‐KO) mice attenuates phenotypic features of slow aging. Aging Cell, 13(6), 981–1000. 10.1111/acel.12262 - DOI - PMC - PubMed
-
- Bae, H. , Gurinovich, A. , Malovini, A. , Atzmon, G. , Andersen, S. L. , Villa, F. , Barzilai, N. , Puca, A. , Perls, T. T. , & Sebastiani, P. (2018). Effects of FOXO3 Polymorphisms on Survival to Extreme Longevity in Four Centenarian Studies. Journals of Gerontology ‐ Series A Biological Sciences and Medical Sciences, 73(11), 1439–1447. 10.1093/gerona/glx124 - DOI - PMC - PubMed
-
- Banasik, K. , Ribel‐Madsen, R. , Gjesing, A. P. , Wegner, L. , Andersson, A. , Poulsen, P. , Borglykke, A. , Witte, D. R. , Pedersen, O. , Hansen, T. , & Vaag, A. (2011). The FOXO3A rs2802292 G‐allele associates with improved peripheral and hepatic insulin sensitivity and increased skeletal muscle‐FOXO3A mRNA expression in twins. The Journal of Clinical Endocrinology and Metabolism, 96(1), E119–E124. 10.1210/jc.2010-0881 - DOI - PubMed
-
- Broer, L. , Buchman, A. S. , Deelen, J. , Evans, D. S. , Faul, J. D. , Lunetta, K. L. , Sebastiani, P. , Smith, J. A. , Smith, A. V. , Tanaka, T. , Yu, L. , Arnold, A. M. , Aspelund, T. , Benjamin, E. J. , De Jager, P. L. , Eirkisdottir, G. , Evans, D. A. , Garcia, M. E. , Hofman, A. , … Murabito, J. M. (2015). GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. Journals of Gerontology ‐ Series A Biological Sciences and Medical Sciences, 70(1), 110–118. 10.1093/gerona/glu166 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

