Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: a double-blind, randomised, placebo-controlled phase 3 clinical trial
- PMID: 36618081
- PMCID: PMC9803910
- DOI: 10.1016/j.lana.2022.100423
Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: a double-blind, randomised, placebo-controlled phase 3 clinical trial
Abstract
Background: SOBERANA-02 is a COVID-19 conjugate vaccine (recombinant RBD conjugated to tetanus toxoid). Phases 1/2 clinical trials demonstrated high immunogenicity, promoting neutralising IgG and specific T-cell response. A third heterologous dose of SOBERANA-Plus (RBD-dimer) further increased neutralising antibodies. The aim of this study is to evaluate the safety and efficacy of two immunisation regimes: two doses of SOBERANA-02 and a heterologous three-dose combination with SOBERANA-Plus added to it.
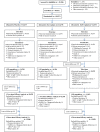
Methods: From March 8th to June 24th, 2021 we conducted in Havana, Cuba a multicentre randomised, double-blind, placebo-controlled, phase-3 trial evaluating a two doses SOBERANA-02 scheme and a heterologous scheme with one dose SOBERANA-Plus added to it (RPCEC00000354). Participants 19-80 years were randomly assigned to receiving 28 days apart either the two or three dose scheme or placebo. The main endpoint was vaccine efficacy in preventing the occurrence of RT-PCR confirmed symptomatic COVID-19 at least 14 days after the second or third dose in the per-protocol population. We also assessed efficacy against severe disease and, in all participants receiving at least one vaccine/placebo dose, safety for 28 days after each dose.
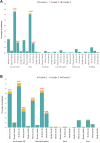
Findings: We included 44,031 participants (52.0% female, 48.0% male; median age 50 years, range 19-80 years; 7.0% black, 24.0% mixed-race, 59.0% white) in a context of initial Beta VOC predominance, with this variant being partially replaced by Delta near the trial's end. Vaccine efficacy in the heterologous combination was 92.0% (95%CI 80.4-96.7) against symptomatic disease. There were no severe COVID-19 cases in the vaccine group against 6 in the placebo group. Two doses of SOBERANA-02 was 69.7% (95%CI 56.5-78.9) and 74.9% (95%CI 33.7-90.5) efficacious against symptomatic and severe COVID-19, respectively. The occurrence of serious and severe adverse events (AE) was very rare and equally distributed between placebo and vaccine groups. Solicited AEs were slightly more frequent in the vaccine group but predominantly local and mostly mild and transient.
Interpretation: Our results indicate that the straightforward to manufacture SOBERANA vaccines are efficacious in a context of Beta and Delta VOC circulation, have a favourable safety profile, and may represent an attractive option for use in COVID-19 vaccination programmes.
Funding: This study received funds from the National Fund for Science and Technology (FONCI-CITMA-Cuba, contract 2020-20) of the Ministry of Science, Technology and Environment of Cuba.
Keywords: COVID-19 vaccine; Conjugate-vaccine; Heterologous scheme; RBD-subunit vaccine; SARS-COV-2; Vaccine efficacy.
© 2022 The Authors.
Conflict of interest statement
VVB, YVB, DGR, YCR, SFC are authors of two patent applications. The other authors declare no competing interests. No author received an honorarium for contributing to this paper.
Figures

References
-
- WHO Vaccine equity. https://www.who.int/campaigns/vaccine-equity
-
- Schneerson R., Robbins J.B., Barrera O., et al. Haemophilus influenzae type B polysaccharide-protein conjugates: model for a new generation of capsular polysaccharide vaccines. Prog Clin Biol Res. 1980;47:77–94. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

