No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment
- PMID: 36618417
- PMCID: PMC9816414
- DOI: 10.3389/fimmu.2022.1075260
No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment
Abstract
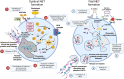
The tumor immune microenvironment (TIME) controls tumorigenesis. Neutrophils are important components of TIME and control tumor progression and therapy resistance. Neutrophil extracellular traps (NETs) ejected by activated neutrophils are net-like structures composed of decondensed extracellular chromatin filaments decorated with a plethora of granules as well as cytoplasmic proteins. Many of these harbour post translational modifications. Cancer cells reportedly trigger NET formation, and conversely, NETs alter the TIME and promote tumor cell proliferation and migration. The specific interactions between NETs and TIME and the respective effects on tumor progression are still elusive. In certain tumors, a CD4+ T helper (Th) 2 cell-associated TIME induces NETs and exerts immunosuppressive functions via programmed death 1 (PD-1)/PD-L1, both associated with poorer prognosis. In other cases, NETs induce the proliferation of Th1 cells, associated with an improved prognosis in cancer. In addition, NETs can drive macrophage polarization and often rely on macrophages to promote cancer cell invasion and metastasis. In turn, macrophages can swiftly clear NETs in an immunologically silent manner. The aim of this review is to summarize the knowledge about the mutual interaction between NETs and TIME and its impact on tumor growth and therapy.
Keywords: adaptive immunity; cancer; immunotherapy; innate immunity; macrophages; neutrophil extracellular traps; neutrophils; tumor microenvironment.
Copyright © 2022 Fang, Stehr, Naschberger, Knopf, Herrmann and Stürzl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

