Developmental pathways linked to the vulnerability of adult midbrain dopaminergic neurons to neurodegeneration
- PMID: 36618829
- PMCID: PMC9815185
- DOI: 10.3389/fnmol.2022.1071731
Developmental pathways linked to the vulnerability of adult midbrain dopaminergic neurons to neurodegeneration
Abstract
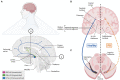
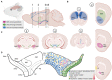
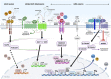
The degeneration of dopaminergic and other neurons in the aging brain is considered a process starting well beyond the infantile and juvenile period. In contrast to other dopamine-associated neuropsychiatric disorders, such as schizophrenia and drug addiction, typically diagnosed during adolescence or young adulthood and, thus, thought to be rooted in the developing brain, Parkinson's Disease (PD) is rarely viewed as such. However, evidences have accumulated suggesting that several factors might contribute to an increased vulnerability to death of the dopaminergic neurons at an already very early (developmental) phase in life. Despite the remarkable ability of the brain to compensate such dopamine deficits, the early loss or dysfunction of these neurons might predispose an individual to suffer from PD because the critical threshold of dopamine function will be reached much earlier in life, even if the time-course and strength of naturally occurring and age-dependent dopaminergic cell death is not markedly altered in this individual. Several signaling and transcriptional pathways required for the proper embryonic development of the midbrain dopaminergic neurons, which are the most affected in PD, either continue to be active in the adult mammalian midbrain or are reactivated at the transition to adulthood and under neurotoxic conditions. The persistent activity of these pathways often has neuroprotective functions in adult midbrain dopaminergic neurons, whereas the reactivation of silenced pathways under pathological conditions can promote the survival and even regeneration of these neurons in the lesioned or aging brain. This article summarizes our current knowledge about signaling and transcription factors involved in midbrain dopaminergic neuron development, whose reduced gene dosage or signaling activity are implicated in a lower survival rate of these neurons in the postnatal or aging brain. It also discusses the evidences supporting the neuroprotection of the midbrain dopaminergic system after the external supply or ectopic expression of some of these secreted and nuclear factors in the adult and aging brain. Altogether, the timely monitoring and/or correction of these signaling and transcriptional pathways might be a promising approach to a much earlier diagnosis and/or prevention of PD.
Keywords: Parkinson’s disease; dopamine; maintenance; mesencephalon; neuroprotection; survival.
Copyright © 2022 Prakash.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Maternal Herpesviridae infection during pregnancy alters midbrain dopaminergic signatures in adult offspring.Neurobiol Dis. 2022 Jul;169:105720. doi: 10.1016/j.nbd.2022.105720. Epub 2022 Apr 11. Neurobiol Dis. 2022. PMID: 35417751
-
Dickkopf 3 Promotes the Differentiation of a Rostrolateral Midbrain Dopaminergic Neuronal Subset In Vivo and from Pluripotent Stem Cells In Vitro in the Mouse.J Neurosci. 2015 Sep 30;35(39):13385-401. doi: 10.1523/JNEUROSCI.1722-15.2015. J Neurosci. 2015. PMID: 26424886 Free PMC article.
-
Similarities and differences between nigral and enteric dopaminergic neurons unravel distinctive involvement in Parkinson's disease.NPJ Parkinsons Dis. 2022 Apr 22;8(1):50. doi: 10.1038/s41531-022-00308-9. NPJ Parkinsons Dis. 2022. PMID: 35459867 Free PMC article. Review.
-
A WNT1-regulated developmental gene cascade prevents dopaminergic neurodegeneration in adult En1(+/-) mice.Neurobiol Dis. 2015 Oct;82:32-45. doi: 10.1016/j.nbd.2015.05.015. Epub 2015 Jun 3. Neurobiol Dis. 2015. PMID: 26049140
-
Molecular Regulation in Dopaminergic Neuron Development. Cues to Unveil Molecular Pathogenesis and Pharmacological Targets of Neurodegeneration.Int J Mol Sci. 2020 Jun 3;21(11):3995. doi: 10.3390/ijms21113995. Int J Mol Sci. 2020. PMID: 32503161 Free PMC article. Review.
Cited by
-
Gene-wide significant association analyses of DNMT1 genetic variants with Parkinson's disease.Front Genet. 2023 Mar 6;14:1112388. doi: 10.3389/fgene.2023.1112388. eCollection 2023. Front Genet. 2023. PMID: 36950137 Free PMC article.
-
Pediatric-Onset Epilepsy and Developmental Epileptic Encephalopathies Followed by Early-Onset Parkinsonism.Int J Mol Sci. 2023 Feb 14;24(4):3796. doi: 10.3390/ijms24043796. Int J Mol Sci. 2023. PMID: 36835207 Free PMC article. Review.
-
TRPV4-mediated Ca2+ deregulation causes mitochondrial dysfunction via the AKT/α-synuclein pathway in dopaminergic neurons.FASEB Bioadv. 2023 Oct 11;5(12):507-520. doi: 10.1096/fba.2023-00057. eCollection 2023 Dec. FASEB Bioadv. 2023. PMID: 38094157 Free PMC article.
-
The Crucial Roles of Pitx3 in Midbrain Dopaminergic Neuron Development and Parkinson's Disease-Associated Neurodegeneration.Int J Mol Sci. 2023 May 11;24(10):8614. doi: 10.3390/ijms24108614. Int J Mol Sci. 2023. PMID: 37239960 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

