Treatment as prevention effect of direct-acting antivirals on primary hepatitis C virus incidence: Findings from a multinational cohort between 2010 and 2019
- PMID: 36618902
- PMCID: PMC9816910
- DOI: 10.1016/j.eclinm.2022.101810
Treatment as prevention effect of direct-acting antivirals on primary hepatitis C virus incidence: Findings from a multinational cohort between 2010 and 2019
Abstract
Background: Broad direct-acting antiviral (DAA) access may reduce hepatitis C virus (HCV) incidence through a "treatment as prevention" (TasP) effect. We assessed changes in primary HCV incidence following DAA access among people living with HIV (PLHIV).
Methods: We used pooled individual-level data from six cohorts from the International Collaboration on Hepatitis C Elimination in HIV Cohorts (InCHEHC). Follow-up started from the first recorded negative HCV antibody test date and ended at last negative antibody test or estimated infection date. Follow-up was restricted to 2010-2019. We used segmented Poisson regression to model trends across pre-, limited- (i.e., restrictions on access) and broad-DAA access periods.
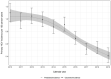
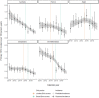
Findings: Overall, 45,942 participants had at least one HCV antibody negative result and follow-up between 2010 and 2019. We observed 2042 incident HCV infections over 248,189 person-years (PY). Pooled incidence decreased from 0.91 per 100 PY in 2015 to 0.41 per 100 PY in 2019. Compared to the average pre-DAA period incidence (0.90 per 100 PY), average incidence was similar during the limited-DAA access period (Incidence rate ratio [IRR] = 0.98; 95%CI = 0.87, 1.11), and 52% lower during the broad-DAA access period (IRR = 0.48; 95%CI = 0.42, 0.52). The average annual decline in HCV incidence was 2% in the pre-DAA period; an additional 9% annual decline in incidence was observed during the limited-DAA access period (IRR = 0.91; 95%CI = 0.82, 1.00) and a further 20% decline in the broad-DAA access period (IRR = 0.80, 95%CI = 0.73, 0.89).
Interpretation: Our findings suggest that broad DAA access has a TasP effect on primary HCV incidence among PLHIV. Based on the initial years of DAA availability, the countries in the InCHEHC collaboration are on track to meet the World Health Organization's 80% HCV incidence reduction target for PLHIV by 2030.
Funding: This study was funded by the Australian Government National Health and Medical Research Council (Grant number GNT1132902).
Keywords: Direct-acting antivirals; Elimination; HIV; Hepatitis C virus; Incidence; Trends.
© 2022 The Authors.
Conflict of interest statement
Juan Berenguer reports honoraria for advice or public speaking from 10.13039/100006483AbbVie, 10.13039/100005564Gilead, 10.13039/100012781MSD, JANSSEN, and 10.13039/100010877ViiV Healthcare; and grants from 10.13039/100006483AbbVie, 10.13039/100005564Gilead, 10.13039/100012781MSD, and 10.13039/100010877ViiV Healthcare. Dominique L Braun reports honoraria for advice or public speaking from, 10.13039/100005564Gilead, 10.13039/100012781MSD and 10.13039/100010877ViiV Healthcare. Marina Klein reports grants for investigator-initiated studies from 10.13039/100010877ViiV Healthcare, 10.13039/100006483AbbVie, 10.13039/100004334Merck, and 10.13039/100005564Gilead, and consulting fees from 10.13039/100010877ViiV Healthcare, 10.13039/100006483AbbVie, and 10.13039/100005564Gilead, all outside the submitted work. Marina Klein is supported by a Tier I Canada Research Chair. Jeffrey V Lazarus acknowledges grants and speaker fees from 10.13039/100006483AbbVie, 10.13039/100005564Gilead Sciences and 10.13039/100012781MSD and speaker fees from Genfit, Intercept, Janssen, 10.13039/100015758Novo Nordisk and 10.13039/100010877ViiV, outside of the submitted work. Andri Rauch reports support to his institution for advisory boards and/or travel grants from 10.13039/100006483Abbvie, 10.13039/100012781MSD, 10.13039/100005564Gilead Sciences, and 10.13039/100004319Pfizer, and an investigator initiated trial (IIT) grant from 10.13039/100005564Gilead Sciences. All remuneration to Andri Rauch went to his home institution and not to Andri Rauch personally, and all remuneration was provided outside the submitted work. Karine Lacombe reports honoraria for advice or public speaking from 10.13039/100006483Abbvie, 10.13039/100005564Gilead, 10.13039/100012781MSD, Janssen and 10.13039/100010877ViiV Healthcare. Fabrice Bonnet reports grants from 10.13039/100005564Gilead and honoraria from 10.13039/100005564Gilead, 10.13039/100005564ViiV healthcare and 10.13039/100012781MSD. Maria Prins reports unrestricted research grants and speaker/advisor fees from 10.13039/100005564Gilead Sciences and 10.13039/100012781MSD; all of which were paid to her institution and unrelated to the current work. Marc van der Valk reports unrestricted research grants from 10.13039/100005564Gilead and 10.13039/100012781MSD and fees for participation in advisory boards from 10.13039/100005564Gilead, 10.13039/100012781MSD and 10.13039/100010877ViiV (all paid to his institution). Joseph S Doyle reports funding to his institution for investigator-initiated research from 10.13039/100005564Gilead Sciences and 10.13039/100006483AbbVIe, and honoraria to his institution for educational events from 10.13039/100006483AbbVie. Linda Wittkop reports grants/financial support for the work under consideration from the French Agency 10.13039/501100003323ANRS Emerging Infectious Diseases (10.13039/501100003323ANRS—MIE) paid to her institution. Gail Matthews reports grants from 10.13039/100005564Gilead, 10.13039/100006483AbbVie and 10.13039/100010877ViiV, all paid to her institution and financial support for participating in advisory board from 10.13039/100005564Gilead and 10.13039/100010877ViiV. The CEASE study is supported by 10.13039/100005567Gilead. Inma Jarrin reports grants from 10.13039/100012781MSD and 10.13039/100010877ViiV healthcare, all paid to her institution, honoraria for lectures/presentations from 10.13039/100005564Gilead and 10.13039/100010877ViiV healthcare, support from 10.13039/100005564Gilead for attending meetings/travel, and support from 10.13039/100005564Gilead to participate in an advisory board. Margaret Hellard reports investigator-initiated research grants from 10.13039/100005564Gilead and 10.13039/100006483Abbvie. Dominique Salmon reports support for attending meetings/travel from 10.13039/100005564Gilead and 10.13039/100006483AbbVie. Mark Stoové reports funding from 10.13039/100005564Gilead and 10.13039/100006483AbbVie for investigator-initiated research unrelated to this work and consultant fees from 10.13039/100005564Gilead Sciences for activities unrelated to this work.
Figures

References
-
- Polaris Observatory HCV Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415. - PubMed
-
- World Health Organization Hepatitis C. 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
-
- Sogni P., Gilbert C., Lacombe K., et al. All-oral direct-acting antiviral regimens in HIV/hepatitis C virus-coinfected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13-HEPAVIH cohort. Clin Infect Dis. 2016;63(6):763–770. - PubMed
-
- World Health Organization . 2021. Interim guidance for country validation of viral hepatitis elimination. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

