TWN-RENCOD: A novel method for protein binding site comparison
- PMID: 36618985
- PMCID: PMC9798139
- DOI: 10.1016/j.csbj.2022.12.014
TWN-RENCOD: A novel method for protein binding site comparison
Abstract
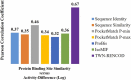
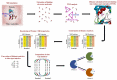
Several diverse proteins possess similar binding sites. Protein binding site comparison provides valuable insights for the drug discovery and development. Binding site similarities are useful in understanding polypharmacology, identifying potential off-targets and repurposing of known drugs. Many binding site analysis and comparison methods are available today, however, these methods may not be adequate to explain variation in the activity of a drug or a small molecule against a number of similar proteins. Water molecules surrounding the protein surface contribute to structure and function of proteins. Water molecules form diverse types of hydrogen-bonded cyclic water-ring networks known as topological water networks (TWNs). Analysis of TWNs in binding site of proteins may improve understanding of the characteristics of binding sites. We propose TWN-based residue encoding (TWN-RENCOD), a novel binding site comparison method which compares the aqueous environment in binding sites of similar proteins. As compared to other existing methods, results obtained using our method correlated better with differences in wide range of activity of a known drug (Sunitinib) against nine different protein kinases (KIT, PDGFRA, VEGFR2, PHKG2, ITK, HPK1, MST3, PAK6 and CDK2).
Keywords: Binding site comparison method; Drug repurposing; Protein binding site; Sunitinib; Water network.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Ehrt C., Brinkjost T., Koch O. Impact of binding site comparisons on medicinal chemistry and rational molecular design. J Med Chem. 2016;59(9):4121–4151. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
