Semi-automated serum steroid profiling with tandem mass spectrometry
- PMID: 36619216
- PMCID: PMC9813517
- DOI: 10.1016/j.jmsacl.2022.12.006
Semi-automated serum steroid profiling with tandem mass spectrometry
Abstract
Objectives: Highly selective and sensitive multi-analyte methods for the analysis of steroids are attractive for the diagnosis of endocrine diseases. Commercially available kits are increasingly used for this purpose. These methods involve laborious solid phase extraction, and the respective panels of target analytes are incomplete. We wanted to investigate whether an improvement of kit solutions is possible by introducing automated on-line solid phase extraction (SPE) and combining originally separate analyte panels.
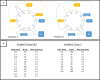
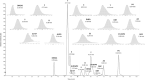
Methods: Sample preparation was performed using automated on-line SPE on a high-pressure stable extraction column. Chromatographic separation, including isobaric compounds, was achieved using a 0.25 mM ammonium fluoride-methanol gradient on a small particle size biphenyl column. Standard compounds and internal standard mixtures of two panels of a commercially available kit were combined to achieve an optimized and straightforward detection of 15 endogenous steroids. Validation was performed according to the European Medicines Agency (EMA) guidelines with slight modifications.
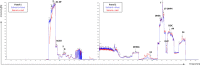
Results: Validation was successfully performed for all steroids over a clinically relevant calibration range. Deviations of intra- and inter-assay accuracy and precision results passed the criteria and no relevant matrix effects were detected due to highly effective sample preparation. External quality assessment samples showed the applicability as a routine diagnostic method, which was affirmed by the analyses of anonymized clinical samples.
Conclusions: It was found possible to complement a commercially available kit for quantitative serum steroid profiling based on isotope dilution LC-MS/MS by implementing automated on-line SPE, thereby improving the practicality and robustness of the measurement procedure.
Keywords: A4, Androstendione; ALDO, Aldosterone; APCI, Atmospheric pressure chemical ionization; CAH, Congenital adrenal hyperplasia; CE, Collision energy; CE-IVD, Certified in-vitro-diagnostic device; CV, Coefficient of variation; DHEA, Dehydro-epiandrosterone; DHEA-S, Dehydro-epiandrosterone sulfate; DHT, Dihydrotestosterone; DOC, 11-deoxycorticosterone; E, Cortisone; E2, Estradiol; EMA, European Medicines Agency; EQA, External quality assessment; ESI, Electrospray ionisation; F, Cortisol; IVD, In-vitro-diagnostic; IVDR, EU In vitro Diagnostic Regulation; LC, Liquid chromatography; LC–MS/MS, Liquid chromatography tandem mass spectrometry; LDT, Laboratory developed test; LLOQ, Lower limit of quantification; MRM, Multiple reaction monitoring; On-line solid phase extraction (SPE); P4, Progesterone; QC, Quality control; Robustness; S/N, Signal-to-noise ratio; SID, Stable-isotope dilution; SPE, Solid phase extraction; SST, System suitability test; Serum steroid profiling; Stable-isotope dilution liquid chromatography-tandem mass spectrometry (SID LC-MS/MS); UHPLC, Ultra high performance liquid chromatography; ULOQ, Upper limit of quantification.
© 2022 THE AUTHORS.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A novel fully-automated method to measure steroids in serum by liquid chromatography-tandem mass spectrometry.J Mass Spectrom Adv Clin Lab. 2022 Dec 15;27:24-32. doi: 10.1016/j.jmsacl.2022.12.004. eCollection 2023 Jan. J Mass Spectrom Adv Clin Lab. 2022. PMID: 36593910 Free PMC article.
-
Multiplexed steroid profiling of gluco- and mineralocorticoids pathways using a liquid chromatography tandem mass spectrometry method.J Steroid Biochem Mol Biol. 2017 Jan;165(Pt B):202-211. doi: 10.1016/j.jsbmb.2016.06.005. Epub 2016 Jun 23. J Steroid Biochem Mol Biol. 2017. PMID: 27339652
-
[Determination of eight environmental phenols in human urine samples by high-throughput solid-phase extraction-ultra-performance liquid chromatography-tandem mass spectrometry].Se Pu. 2020 Dec 8;38(12):1456-1464. doi: 10.3724/SP.J.1123.2020.07021. Se Pu. 2020. PMID: 34213261 Chinese.
-
Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: could it raise new perspectives in the diagnostic field of hormone-dependent malignancies?J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 1;940:24-34. doi: 10.1016/j.jchromb.2013.09.022. Epub 2013 Sep 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 24140653 Review.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Assessment of ammonium fluoride as a mobile phase additive for sensitivity gains in electrospray ionization.Anal Sci Adv. 2023 Oct 12;4(11-12):347-354. doi: 10.1002/ansa.202300031. eCollection 2023 Dec. Anal Sci Adv. 2023. PMID: 38715648 Free PMC article.
References
-
- Payne A.H., Hales D.B. Overview of Steroidogenic Enzymes in the Pathway from Cholesterol to Active Steroid Hormones. Endocr. Rev. 2004;25(6):947–970. - PubMed
-
- Vogeser M., Seger C. Pitfalls Associated with the Use of Liquid Chromatography-Tandem Mass Spectrometry in the Clinical Laboratory. Clin. Chem. 2010;56(8):1234–1244. - PubMed
-
- Marcos J., Pozo O.J. Current LC–MS methods and procedures applied to the identification of new steroid metabolites. J. Steroid Biochem. Mol. Biol. 2016;162:41–56. - PubMed
-
- Di Dalmazi G., Fanelli F., Mezzullo M., Casadio E., Rinaldi E., Garelli S., Giampalma E., Mosconi C., Golfieri R., Vicennati V., Pagotto U., Pasquali R. Steroid Profiling by LC-MS/MS in Nonsecreting and Subclinical Cortisol-Secreting Adrenocortical Adenomas. J. Clin. Endocrinol. Metab. 2015;100(9):3529–3538. - PubMed
-
- Janzen N., Peter M., Sander S., Steuerwald U., Terhardt M., Holtkamp U., et al. Newborn Screening for Congenital Adrenal Hyperplasia: Additional Steroid Profile using Liquid Chromatography-Tandem Mass Spectrometry. J. Clin. Endocrinol. Metab. 2007;92(7):2581–2589. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

