Taisho-Sanshoku koi have hardly faded skin and show attenuated melanophore sensitivity to adrenaline and melanin-concentrating hormone
- PMID: 36619537
- PMCID: PMC9813866
- DOI: 10.3389/fendo.2022.994060
Taisho-Sanshoku koi have hardly faded skin and show attenuated melanophore sensitivity to adrenaline and melanin-concentrating hormone
Abstract
Introduction: Koi carp, an ornamental fish derived from the common carp Cyprinus carpio (CC), is characterized by beautiful skin color patterns. However, the mechanism that gives rise to the characteristic vivid skin coloration of koi carp has not been clarified. The skin coloration of many teleosts changes in response to differences in the background color. This change in skin coloration is caused by diffusion or aggregation of pigment granules in chromatophores and is regulated mainly by sympathetic nerves and hormones. We hypothesized that there would be some abnormality in the mechanism of skin color regulation in koi carp, which impairs skin color fading in response to background color.
Methods: We compared the function of melanin-concentrating hormone (MCH), noradrenaline, and adrenaline in CC and Taisho-Sanshoku (TS), a variety of tri-colored koi.
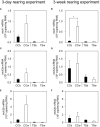
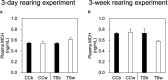
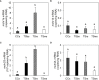
Results and discussion: In CC acclimated to a white background, the skin color became paler and pigment granules aggregated in melanophores in the scales compared to that in black-acclimated CC. There were no clear differences in skin color or pigment granule aggregation in white- or black-acclimated TS. The expression of mch1 mRNA in the brain was higher in the white-acclimated CC than that in the black-acclimated CC. However, the expression of mch1 mRNA in the brain in the TS did not change in response to the background color. Additionally, plasma MCH levels did not differ between white- and black-acclimated fish in either CC or TS. In vitro experiments showed that noradrenaline induced pigment aggregation in scale melanophores in both CC and TS, whereas adrenaline induced pigment aggregation in the CC but not in the TS. In vitro administration of MCH induced pigment granule aggregation in the CC but not in the TS. However, intraperitoneal injection of MCH resulted in pigment granule aggregation in both CC and TS. Collectively, these results suggest that the weak sensitivity of scale melanophores to MCH and adrenaline might be responsible for the lack of skin color change in response to background color in the TS.
Keywords: background color; catecholamine; koi carp; melanin-concentrating hormone; melanophore; skin color.
Copyright © 2022 Shinohara, Kasagi, Amiya, Hoshino, Ishii, Hyodo, Yamaguchi, Sato, Amano, Takahashi and Mizusawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Takahashi A, Mizusawa K, Amano M. Multifunctional roles of melanocyte-stimulating hormone and melanin-concentrating hormone in fish: Evolution from classical body color change. Aqua-BioSci Monogr (2014) 7:1–46. doi: 10.5047/absm.2014.00701.0001 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

