The mechanistic role of neutrophil lymphocyte ratio perturbations in the leading non communicable lifestyle diseases
- PMID: 36619602
- PMCID: PMC9780608
- DOI: 10.12688/f1000research.123245.1
The mechanistic role of neutrophil lymphocyte ratio perturbations in the leading non communicable lifestyle diseases
Abstract
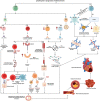
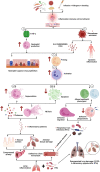
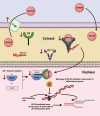
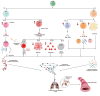
Inflammation plays a critical role in the development and progression of chronic diseases like type 2 diabetes mellitus, coronary artery disease, and chronic obstructive pulmonary disease. Inflammatory responses are indispensable for pathogen control and tissue repair, but they also cause collateral damage. A chronically activated immune system and the resultant immune dysregulation mediated inflammatory surge may cause multiple negative effects, requiring tight regulation and dampening of the immune response to minimize host injury. While chronic diseases are characterized by systemic inflammation, the mechanistic relationship of neutrophils and lymphocytes to inflammation and its correlation with the clinical outcomes is yet to be elucidated. The neutrophil to lymphocyte ratio (NLR) is an easy-to-measure laboratory marker used to assess systemic inflammation. Understanding the mechanisms of NLR perturbations in chronic diseases is crucial for risk stratification, early intervention, and finding novel therapeutic targets. We investigated the correlation between NLR and prevalent chronic conditions as a measure of systemic inflammation. In addition to predicting the risk of impending chronic conditions, NLR may also provide insight into their progression. This review summarizes the mechanisms of NLR perturbations at cellular and molecular levels, and the key inflammatory signaling pathways involved in the progression of chronic diseases. We have also explored preclinical studies investigating these pathways and the effect of quelling inflammation in chronic disease as reported by a few in vitro, in vivo studies, and clinical trials.
Keywords: Neutrophil-lymphocyte ratio; chronic obstructive pulmonary disease; coronary artery disease; inflammation; non communicable diseases; type 2 diabetes mellitus.
Copyright: © 2022 Biswas M et al.
Conflict of interest statement
No competing interests were disclosed.
Figures


References
-
- World Health Organisation: Noncommunicable diseases. Geneva: WHO;1948. 2021 Apr 12 [cited 2022 Apr 15]; [about 5 screens]. Reference Source
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

