Canakinumab as first-line biological therapy in Still's disease and differences between the systemic and the chronic-articular courses: Real-life experience from the international AIDA registry
- PMID: 36619631
- PMCID: PMC9813488
- DOI: 10.3389/fmed.2022.1071732
Canakinumab as first-line biological therapy in Still's disease and differences between the systemic and the chronic-articular courses: Real-life experience from the international AIDA registry
Abstract
Objective: Interleukin (IL)-1 inhibitors are largely employed in patients with Still's disease; in cases with refractory arthritis, IL-6 inhibitors have shown to be effective on articular inflammatory involvement. The aim of the present study is to assess any difference in the effectiveness of the IL-1β antagonist canakinumab prescribed as first-line biologic agent between the systemic and the chronic-articular Still's disease.
Methods: Data were drawn from the retrospective phase of the AutoInflammatory Disease Alliance (AIDA) international registry dedicated to Still's disease. Patients with Still's disease classified according to internationally accepted criteria (Yamaguchi criteria and/or Fautrel criteria) and treated with canakinumab as first-line biologic agent were enrolled.
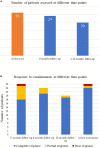
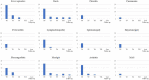
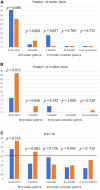
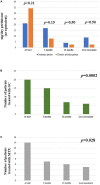
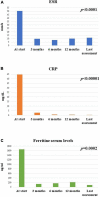
Results: A total of 26 patients (17 females, 9 males; 18 patients developing Still's disease after the age of 16 years) were enrolled; 16 (61.5%) patients suffered from the systemic pattern of the disease; 10 (38.5%) patients suffered from the chronic-articular type. No differences were observed between the systemic and the chronic-articular Still's disease in the frequency of complete response, of flares after the start of canakinumab (p = 0.701) and in the persistence in therapy (p = 0.62). No statistical differences were observed between the two groups after 3 months, 12 months and at the last assessment in the decrease of: the systemic activity score (p = 0.06, p = 0.17, p = 0.17, respectively); the disease activity score on 28 joints (p = 0.54, p = 0.77, p = 0.98, respectively); the glucocorticoid dosage (p = 0.15, p = 0.50, and p = 0.50, respectively); the use of concomitant disease modifying anti-rheumatic drugs (p = 0.10, p = 1.00, and p = 1.00, respectively). No statistically significant differences were observed in the decrease of erythrocyte sedimentation rate (p = 0.34), C reactive protein (p = 0.48), and serum ferritin levels (p = 0.34) after the start of canakinumab.
Conclusion: Canakinumab used for Still's disease has been effective in controlling both clinical and laboratory manifestations disregarding the type of disease course when used as first-line biotechnological agent. These excellent results might have been further enhanced by the early start of IL-1 inhibition.
Keywords: AOSD; adult onset Still’s disease; autoinflammatory diseases; biological therapy; interleukin-1; sJIA; systemic juvenile idiopathic arthritis.
Copyright © 2022 Vitale, Caggiano, Maggio, Lopalco, Emmi, Sota, La Torre, Ruscitti, Bartoloni, Conti, Fabiani, Mattioli, Gaggiano, Cardinale, Dagna, Campochiaro, Giacomelli, Balistreri, Laskari, Tufan, Ragab, Almaghlouth, Więsik-Szewczyk, Pereira, Frediani, Iannone, Sfikakis and Cantarini.
Conflict of interest statement
Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: LC, Novartis. PR, Sobi, Abbvie, Novartis Ely Lilly, and BMS. CC, Sobi Novartis, Jannsen, and Boehringer. EW-S, Novartis and Sobi. LD, Novartis, Sobi, Roche, Galapagos, Jannsen, and Pfizer. PS, Abbvie, UCB, Novartis, Lilly, Genesis, Enorasis, Amgen, and Yansen. Support for attending meetings and travel: CC, Novartis and Amgen. EW-S, Sobi. LD, Novartis. Participation on a Data Safety Monitoring Board or Advisory Board: CC, Boehringer. LD, Novartis. Consulting fees: LD, Novartis, SOBI, Roche, Galapagos, Jannsen, and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Nirmala N, Brachat A, Feist E, Blank N, Specker C, Witt M, et al. Gene-expression analysis of adult-onset still’s disease and systemic juvenile idiopathic arthritis is consistent with a continuum of a single disease entity. Pediatr Rheumatol Online J. (2015) 13:50. 10.1186/s12969-015-0047-3 - DOI - PMC - PubMed
-
- Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult still’s disease. J Rheumatol. (1992) 19:424–30. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

