Loss of TMEM106B exacerbates C9ALS/FTD DPR pathology by disrupting autophagosome maturation
- PMID: 36619668
- PMCID: PMC9812496
- DOI: 10.3389/fncel.2022.1061559
Loss of TMEM106B exacerbates C9ALS/FTD DPR pathology by disrupting autophagosome maturation
Abstract
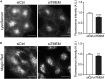
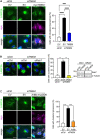
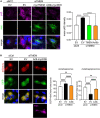
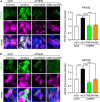
Disruption to protein homeostasis caused by lysosomal dysfunction and associated impairment of autophagy is a prominent pathology in amyotrophic lateral sclerosis and frontotemporal dementia (ALS/FTD). The most common genetic cause of ALS/FTD is a G4C2 hexanucleotide repeat expansion in C9orf72 (C9ALS/FTD). Repeat-associated non-AUG (RAN) translation of G4C2 repeat transcripts gives rise to dipeptide repeat (DPR) proteins that have been shown to be toxic and may contribute to disease etiology. Genetic variants in TMEM106B have been associated with frontotemporal lobar degeneration with TDP-43 pathology and disease progression in C9ALS/FTD. TMEM106B encodes a lysosomal transmembrane protein of unknown function that is involved in various aspects of lysosomal biology. How TMEM106B variants affect C9ALS/FTD is not well understood but has been linked to changes in TMEM106B protein levels. Here, we investigated TMEM106B function in the context of C9ALS/FTD DPR pathology. We report that knockdown of TMEM106B expression exacerbates the accumulation of C9ALS/FTD-associated cytotoxic DPR proteins in cell models expressing RAN-translated or AUG-driven DPRs as well as in C9ALS/FTD-derived iAstrocytes with an endogenous G4C2 expansion by impairing autophagy. Loss of TMEM106B caused a block late in autophagy by disrupting autophagosome to autolysosome maturation which coincided with impaired lysosomal acidification, reduced cathepsin activity, and juxtanuclear clustering of lysosomes. Lysosomal clustering required Rab7A and coincided with reduced Arl8b-mediated anterograde transport of lysosomes to the cell periphery. Increasing Arl8b activity in TMEM106B-deficient cells not only restored the distribution of lysosomes, but also fully rescued autophagy and DPR protein accumulation. Thus, we identified a novel function of TMEM106B in autophagosome maturation via Arl8b. Our findings indicate that TMEM106B variants may modify C9ALS/FTD by regulating autophagic clearance of DPR proteins. Caution should therefore be taken when considering modifying TMEM106B expression levels as a therapeutic approach in ALS/FTD.
Keywords: ALS/FTD; C9orf72; DPR; TMEM106B; autophagy.
Copyright © 2022 Bauer, Webster, Shaw, Kok, Castelli, Lin, Smith, Illanes-Álvarez, Higginbottom, Shaw, Azzouz, Ferraiuolo, Hautbergue, Grierson and De Vos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abramoff M. D., Magalhães P. J., Ram S. J. (2004). Image processing with imageJ. Biophotonics Int. 11 36–42.
Grants and funding
- MR/R024162/1/MRC_/Medical Research Council/United Kingdom
- MR/S025979/1/MRC_/Medical Research Council/United Kingdom
- MR/V030140/1/MRC_/Medical Research Council/United Kingdom
- DEVOS/OCT13/870-792/MNDA_/Motor Neurone Disease Association/United Kingdom
- MR/V000470/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

