Understanding laterality disorders and the left-right organizer: Insights from zebrafish
- PMID: 36619867
- PMCID: PMC9816872
- DOI: 10.3389/fcell.2022.1035513
Understanding laterality disorders and the left-right organizer: Insights from zebrafish
Abstract
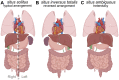
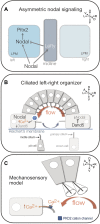
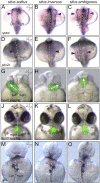
Vital internal organs display a left-right (LR) asymmetric arrangement that is established during embryonic development. Disruption of this LR asymmetry-or laterality-can result in congenital organ malformations. Situs inversus totalis (SIT) is a complete concordant reversal of internal organs that results in a low occurrence of clinical consequences. Situs ambiguous, which gives rise to Heterotaxy syndrome (HTX), is characterized by discordant development and arrangement of organs that is associated with a wide range of birth defects. The leading cause of health problems in HTX patients is a congenital heart malformation. Mutations identified in patients with laterality disorders implicate motile cilia in establishing LR asymmetry. However, the cellular and molecular mechanisms underlying SIT and HTX are not fully understood. In several vertebrates, including mouse, frog and zebrafish, motile cilia located in a "left-right organizer" (LRO) trigger conserved signaling pathways that guide asymmetric organ development. Perturbation of LRO formation and/or function in animal models recapitulates organ malformations observed in SIT and HTX patients. This provides an opportunity to use these models to investigate the embryological origins of laterality disorders. The zebrafish embryo has emerged as an important model for investigating the earliest steps of LRO development. Here, we discuss clinical characteristics of human laterality disorders, and highlight experimental results from zebrafish that provide insights into LRO biology and advance our understanding of human laterality disorders.
Keywords: birth defects; cilia; heterotaxy syndrome; left-right asymmetry; left-right organizer; organ laterality; situs inversus; zebrafish.
Copyright © 2022 Forrest, Barricella, Pohar, Hinman and Amack.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

