Autologous blood resuscitation for large animals in a research setting using the Hemafuse device: Preliminary data of device use for controlled and real-world hemorrhage
- PMID: 36619944
- PMCID: PMC9814117
- DOI: 10.3389/fvets.2022.1069420
Autologous blood resuscitation for large animals in a research setting using the Hemafuse device: Preliminary data of device use for controlled and real-world hemorrhage
Abstract
Introduction: New low-cost technologies are needed to salvage lost blood in low-resource settings and large animal laboratories. The Hemafuse device is a simple mechanical device that can recover lost blood during surgery. The aim of this study is to assess the feasibility of this device for resuscitating large animals with controlled and unintended hemorrhage and to provide device considerations for use in this context.
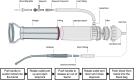
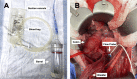
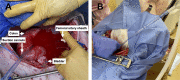
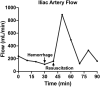
Methods: This study had two experimental components: (1) the Hemafuse device was kept on-shelf and used as needed to assess real-world use for unintended hemorrhage during experiments, and (2) animals underwent a controlled hemorrhage protocol, where four anesthetized swine underwent aortic and external jugular vein catheterization for pressure monitoring. Animals were hemorrhaged into the pelvis, and the Hemafuse device was used to suction the blood through a filter and pushed into a heparinized bag for subsequent retransfusion. Blood samples were collected at baseline, hemorrhage, within the device, and post-retransfusion and laboratory tests were performed.
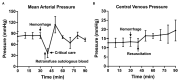
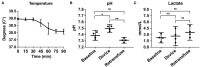
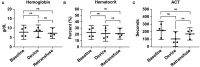
Results: Animals that underwent controlled hemorrhage had a baseline mean arterial pressure of 83.6 ± 7.8 mmHg, and central venous pressure of 12.8 ± 1.9 mmHg, with expected changes throughout hemorrhage and resuscitation. Following resuscitation, pH was similar to baseline (7.39 ± 0.05 vs. 7.31 ± 0.03, p = 0.24). Lactate increased throughout the experiment with no significant differences after autotransfusion compared to baseline (2.7 ± 0.7 vs. 4.1 ± 1.4 mmol/L, p = 0.37). There were no significant changes in metabolic physiology. Compared to baseline, the hemoglobin (7.8 ± 2.4 vs. 7.3 ± 1.8 g/dL, p = 0.74), hematocrit (23% ± 6.9 vs. 21.3% ± 5.6, p = 0.71), and activated clotting time (268.5 ± 44.5 vs. 193 ± 24.6 s, p = 0.35) were similar after retransfusion. When used for unintended hemorrhage, the animals were resuscitated using the device with a mean time to retransfusion time of 128.7 ± 13.3 s and 100% survival throughout the experiment.
Conclusion: The Hemafuse device is feasible and efficacious for supporting large animal resuscitation. This is preliminary evidence that the device is a low-risk and low-cost off-the-shelf option for resuscitation using autologous blood with no significant effect on physiology post-retransfusion. We recommend that research laboratories consider the Hemafuse device for emergency use, particularly for highly invasive surgical laboratories where banked blood is not readily available.
Keywords: Hemafuse; autologous blood; blood bank; hemorrhage; large animals; resuscitation; whole blood.
Copyright © 2022 Treffalls, Lubas, Morrison and Stonko.
Conflict of interest statement
DS receives payments from Catalio Capital Management LP for investment analysis. Sisu Global Health is a Catalio portfolio company. ML is an employee of Sisu Global Health. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Low-volume resuscitation with a polymerized bovine hemoglobin-based oxygen-carrying solution (HBOC-201) provides adequate tissue oxygenation for survival in a porcine model of controlled hemorrhage.J Trauma. 2003 Nov;55(5):873-85. doi: 10.1097/01.TA.0000092681.17874.6F. J Trauma. 2003. PMID: 14608160
-
Evaluation of the effects of autotransfusion of unprocessed blood on hemodynamics and oxygen transport in anesthetized pigs.Crit Care Med. 1996 May;24(5):855-61. doi: 10.1097/00003246-199605000-00021. Crit Care Med. 1996. PMID: 8706465
-
MalPEG-hemoglobin (MP4) improves hemodynamics, acid-base status, and survival after uncontrolled hemorrhage in anesthetized swine.Crit Care Med. 2005 Aug;33(8):1794-804. doi: 10.1097/01.ccm.0000172648.55309.13. Crit Care Med. 2005. PMID: 16096458
-
A prospective, randomized trial of intravenous hydroxocobalamin versus whole blood transfusion compared to no treatment for Class III hemorrhagic shock resuscitation in a prehospital swine model.Acad Emerg Med. 2015 Mar;22(3):321-30. doi: 10.1111/acem.12605. Epub 2015 Mar 2. Acad Emerg Med. 2015. PMID: 25731610
-
Continuous fiberoptic arterial and venous blood gas monitoring in hemorrhagic shock.Chest. 1996 Apr;109(4):1049-55. doi: 10.1378/chest.109.4.1049. Chest. 1996. PMID: 8635330
References
-
- Treffalls RN, Stonko DP, Edwards J, Abdou H, Savidge SG, Walker P, et al. . Characterization of the mesenteric circulatory physiology during hemorrhagic shock in a swine model. Surg Pract Sci. (2022) 25:100119. 10.1016/j.sipas.2022.100119 - DOI
-
- Stonko DP, Edwards J, Abdou H, Elansary N, Lang E, Savidge SG, et al. . Quantifying the cardiovascular physiology of REBOA and partial REBOA: how REBOA facilitates resuscitation but also strains the left ventricle. J Vasc Surg. (2022) 75:e317–8. 10.1016/j.jvs.2022.03.748 - DOI
LinkOut - more resources
Full Text Sources

