The prelimbic cortex regulates itch processing by controlling attentional bias
- PMID: 36619983
- PMCID: PMC9816985
- DOI: 10.1016/j.isci.2022.105829
The prelimbic cortex regulates itch processing by controlling attentional bias
Abstract
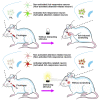
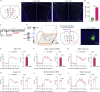
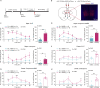
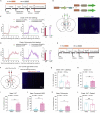
Itch is a complex and unpleasant sensory experience. Recent studies have begun to investigate the neural mechanisms underlying the modulation of sensory and emotional components of itch in the brain. However, the key brain regions and neural mechanism involved in modulating the attentional processing of itch remain elusive. Here, we showed that the prelimbic cortex (PrL) is associated with itch processing and that the manipulation of itch-responsive neurons in the PrL significantly disrupted itch-induced scratching. Interestingly, we found that increasing attentional bias toward a distracting stimulus could disturb itch processing. We also demonstrated the existence of a population of attention-related neurons in the PrL that drive attentional bias to regulate itch processing. Importantly, itch-responsive neurons and attention-related neurons significantly overlapped in the PrL and were mutually interchangeable in the regulation of itch processing at the cellular activity level. Our results revealed that the PrL regulates itch processing by controlling attentional bias.
Keywords: Biological sciences; Cell biology; Neuroscience.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ikoma A., Steinhoff M., Ständer S., Yosipovitch G., Schmelz M. The neurobiology of itch. Nat. Rev. Neurosci. 2006;7:535–547. - PubMed
-
- Dhand A., Aminoff M.J. The neurology of itch. Brain. 2014;137:313–322. - PubMed
-
- Ikoma A. Updated neurophysiology of itch. Biol. Pharm. Bull. 2013;36:1235–1240. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

