Establishment and evaluation of an in vitro blast lung injury model using alveolar epithelial cells
- PMID: 36620304
- PMCID: PMC9816474
- DOI: 10.3389/fpubh.2022.994670
Establishment and evaluation of an in vitro blast lung injury model using alveolar epithelial cells
Abstract
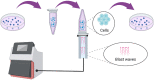
Background: Gas explosion is a fatal disaster commonly occurred in coal mining and often causes systematic physical injuries, of which blast lung injury is the primary one and has not yet been fully investigated due to the absence of disease models. To facilitate studies of this field, we constructed an in vitro blast lung injury model using alveolar epithelial cells.
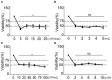
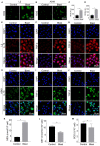
Methods: We randomly divided the alveolar epithelial cells into the control group and blast wave group, cells in the blast wave group were stimulated with different strengths of blast wave, and cells in the control group received sham intervention. Based on the standards we set up for a successful blast injury model, the optimal modeling conditions were studied on different frequencies of blast wave, modeling volume, cell incubation duration, and cell density. The changes of cell viability, apoptosis, intracellular oxidative stress, and inflammation were measured.
Results: We found that cell viability decreased by approximately 50% at 6 h after exposing to 8 bar energy of blast wave, then increased with the extension of culture time and reached to (74.33 ± 9.44) % at 12 h. By applying 1000 ~ 2500 times of shock wave to 1 ~ 5 × 105 cells /ml, the changes of cell viability could well meet the modeling criteria. In parallel, the content of reactive oxide species (ROS), malonaldehyde (MDA), interleukin 18 (IL-18), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β) increased in the blast wave group, while superoxide dismutase (SOD) and Glutathione -S- transferase (GST) decreased, which were highly consistent with that of human beings with gas explosion-induced pulmonary injury.
Conclusion: An in vitro blast lung injury model is set up using a blast wave physiotherapy under 8 bar, 10 Hz blast wave on (1 ~ 5) ×105 alveolar epithelial cells for 1 000 times. This model is flexible, safe, and stable, and can be used for studies of lung injury caused by gas explosion and blast-associated other external forces.
Keywords: alveolar epithelial cells; blast injury; gas explosion; lung injury model; oxidative stress response.
Copyright © 2022 Ding, Hong, Zhang, Sun, Li, Zhang, Ma, Tian, Ren, Zhang and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Shock waves increase pulmonary vascular leakage, inflammation, oxidative stress, and apoptosis in a mouse model.Exp Biol Med (Maywood). 2018 Jul;243(11):934-944. doi: 10.1177/1535370218784539. Epub 2018 Jul 8. Exp Biol Med (Maywood). 2018. PMID: 29984607 Free PMC article.
-
Perfluorocarbon reduces cell damage from blast injury by inhibiting signal paths of NF-κB, MAPK and Bcl-2/Bax signaling pathway in A549 cells.PLoS One. 2017 Mar 21;12(3):e0173884. doi: 10.1371/journal.pone.0173884. eCollection 2017. PLoS One. 2017. PMID: 28323898 Free PMC article.
-
NF-κB and FosB mediate inflammation and oxidative stress in the blast lung injury of rats exposed to shock waves.Acta Biochim Biophys Sin (Shanghai). 2021 Mar 2;53(3):283-293. doi: 10.1093/abbs/gmaa179. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 33677486
-
Vaporized perfluorocarbon inhalation attenuates primary blast lung injury in canines by inhibiting mitogen-activated protein kinase/nuclear factor-κB activation and inducing nuclear factor, erythroid 2 like 2 pathway.Toxicol Lett. 2020 Feb 1;319:49-57. doi: 10.1016/j.toxlet.2019.10.019. Epub 2019 Nov 3. Toxicol Lett. 2020. PMID: 31693926
-
[Experimental study on acute respiratory distress syndrome and analysis of relevant factors in rabbits subjected to thoracic blast trauma].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011 Apr;23(4):243-6. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011. PMID: 21473830 Chinese.
Cited by
-
Esophageal pressure monitoring and its clinical significance in severe blast lung injury.Front Bioeng Biotechnol. 2024 May 9;12:1280679. doi: 10.3389/fbioe.2024.1280679. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38784763 Free PMC article.
-
Age-related exacerbation of lung damage after trauma is associated with increased expression of inflammasome components.Front Immunol. 2024 Jan 11;14:1253637. doi: 10.3389/fimmu.2023.1253637. eCollection 2023. Front Immunol. 2024. PMID: 38274788 Free PMC article.
-
Evaluating the effectiveness of handheld ultrasound in primary blast lung injury: a comprehensive study.Sci Rep. 2025 Jan 18;15(1):2358. doi: 10.1038/s41598-025-86928-6. Sci Rep. 2025. PMID: 39824921 Free PMC article.
-
Assessment model of blast injury: A narrative review.iScience. 2025 Jun 6;28(7):112830. doi: 10.1016/j.isci.2025.112830. eCollection 2025 Jul 18. iScience. 2025. PMID: 40612508 Free PMC article. Review.
-
Pathophysiological changes and injury markers for acute lung injury from blunt impact in infant rabbits.Front Pediatr. 2024 Jun 7;12:1354531. doi: 10.3389/fped.2024.1354531. eCollection 2024. Front Pediatr. 2024. PMID: 38910959 Free PMC article.
References
-
- Wei Q, Hu B, Fang H, Zheng C, Hou X, Gao D, et al. . Effective approach with extra desorption time to estimate the gas content of deep-buried coalbed methane reservoirs: a case study from the Panji Deep Area in Huainan Coalfield, China. ACS Omega. (2022) 7:11240–51. 10.1021/acsomega.2c00142 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

