Case report: 18F-FES PET/CT predicted treatment responses of second-line and third-line CDK4/6 inhibitors after disease progression on first-line CDK4/6 inhibitor in a HR+/HER2- metastatic breast cancer patient
- PMID: 36620595
- PMCID: PMC9816999
- DOI: 10.3389/fonc.2022.1095779
Case report: 18F-FES PET/CT predicted treatment responses of second-line and third-line CDK4/6 inhibitors after disease progression on first-line CDK4/6 inhibitor in a HR+/HER2- metastatic breast cancer patient
Abstract
Background: Cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) has become the commonest first-line treatment of hormonal receptor positive and human epidermal growth factor receptor 2 negative (HR+/HER2-) metastatic breast cancer (MBC). However, therapy is quite individualized after progression of disease (PD) when CDK4/6i fails. Estrogen receptor (ER) status of metastatic lesions of bone, lung or liver might be different from the primary tumor and biopsy of metastatic lesions was invasive and not always available. Prediction of treatment response after PD of CDK4/6i remains unsolved. 18F-fluoroestradiol (FES) PET/CT could non-invasively reveal ER expression both in primary and metastatic breast cancer and recognize heterogeneity of ER status.
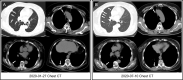
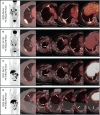
Case presentation: A 70-year-old woman with Parkinson's disease, osteoporosis and cardiovascular co-morbidity was diagnosed with HR+/HER2- breast cancer (pT2N2M0, stage IIIa). Three years later, she developed metastases in right lung and pleura with pleural effusion and received palbociclib + letrozole. After 8 months the disease progressed, and 18F-FES PET/CT revealed multiple ER-positive pleural lesions and ER-negative pulmonary nodules after PD and the progression-free survival (PFS) of first-line CDK4/6i was 8 months. Since most of the metastatic lesions were ER-positive, abemaciclib + fulvestrant were chosen as the second-line CDK4/6i treatment and the PFS was 15 months. Another 18F-FES PET/CT showed a new ER-positive pleural mass with multiple ER-negative pulmonary nodules. Since 18F-FES PET/CT revealed that the dominant lesions were still ER-positive, dalpiciclib + exemestane + fulvestrant were prescribed as the third-line CDK4/6i treatment. Currently the patient's disease had been stable for 2 months.
Conclusion: This case demonstrated that 18F-FES PET/CT could show ER heterogeneity non-invasively and reveal the treatment responses a predictive imaging tool of serial second- and third-line of CDK4/6i treatments when first-line CDK4/6i failed in HR+/HER2- MBC. So long as the dominant or newly-developed metastatic lesion was ER-positive on 18F-FES PET after first-line CDK4/6i, the patient might show certain therapeutic response towards endocrine-based treatment including second- and third-line of CDK4/6i, and thus increased the time to chemotherapy (TTC).
Keywords: CDK4/6 inhibitor; FES PET/CT; estrogen receptor (ER); metastatic breast cancer; treatment response.
Copyright © 2022 Pan, Hao, Xu, Wang, Yao, Wang, Ren, Zhou, Sun and Huo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewers JL and FM declared a shared parent affiliation with the authors to the handling editor at the time of review.
Figures

Similar articles
-
18F-fluoroestradiol (18F-FES) PET/CT for guiding first-line treatment in patients with HR + /HER2- metastatic breast cancer: impact on progression free survival.Eur J Nucl Med Mol Imaging. 2025 Jul 19. doi: 10.1007/s00259-025-07459-w. Online ahead of print. Eur J Nucl Med Mol Imaging. 2025. PMID: 40682675
-
Real-world comparison of palbociclib, abemaciclib, and dalpiciclib as first-line treatments for Chinese HR+/HER2-metastatic breast cancer patients: a multicenter study (YOUNGBC-28).Ther Adv Med Oncol. 2024 Dec 17;16:17588359241302018. doi: 10.1177/17588359241302018. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39697620 Free PMC article.
-
Real-world Outcomes of Cyclin-dependent Kinase Inhibitors Continued Beyond First Disease Progression in Hormone Receptor-positive Metastatic Breast Cancer.Clin Breast Cancer. 2021 Jun;21(3):205-209. doi: 10.1016/j.clbc.2020.09.010. Epub 2020 Oct 2. Clin Breast Cancer. 2021. PMID: 33189562
-
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i.BMC Cancer. 2024 May 23;24(1):631. doi: 10.1186/s12885-024-12269-8. BMC Cancer. 2024. PMID: 38783218 Free PMC article.
-
Cyclin-Dependent Kinase 4/6 Inhibitors for Treatment of Hormone Receptor-Positive, ERBB2-Negative Breast Cancer: A Review.JAMA Oncol. 2023 Sep 1;9(9):1273-1282. doi: 10.1001/jamaoncol.2023.2000. JAMA Oncol. 2023. PMID: 37382948 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

