Returning integrated genomic risk and clinical recommendations: The eMERGE study
- PMID: 36621880
- PMCID: PMC10085845
- DOI: 10.1016/j.gim.2023.100006
Returning integrated genomic risk and clinical recommendations: The eMERGE study
Abstract
Purpose: Assessing the risk of common, complex diseases requires consideration of clinical risk factors as well as monogenic and polygenic risks, which in turn may be reflected in family history. Returning risks to individuals and providers may influence preventive care or use of prophylactic therapies for those individuals at high genetic risk.
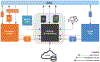
Methods: To enable integrated genetic risk assessment, the eMERGE (electronic MEdical Records and GEnomics) network is enrolling 25,000 diverse individuals in a prospective cohort study across 10 sites. The network developed methods to return cross-ancestry polygenic risk scores, monogenic risks, family history, and clinical risk assessments via a genome-informed risk assessment (GIRA) report and will assess uptake of care recommendations after return of results.
Results: GIRAs include summary care recommendations for 11 conditions, education pages, and clinical laboratory reports. The return of high-risk GIRA to individuals and providers includes guidelines for care and lifestyle recommendations. Assembling the GIRA required infrastructure and workflows for ingesting and presenting content from multiple sources. Recruitment began in February 2022.
Conclusion: Return of a novel report for communicating monogenic, polygenic, and family history-based risk factors will inform the benefits of integrated genetic risk assessment for routine health care.
Trial registration: ClinicalTrials.gov NCT05277116.
Keywords: Common variants; Family history; Genotyping; Monogenic risks; Polygenic risk scores.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest N.S.A.-H. is an employee and equity holder of 23andMe; serves as a scientific advisory board member for Allelica, Inc; received personal fees from Genentech Inc, Allelica Inc, and 23andMe; received research funding from Akcea Therapeutics; and was previously employed by Regeneron Pharmaceuticals. T.W. has grant funding from Gilead Sciences, Inc. L.O. and T.R.-B are founders of a company developing MeTree. T.S., E.D.E., and E.H. are employees and stockholders of Invitae Corporation. E.M.M. has been a consultant for Avidity Bioscience, Amgen Inc, AstraZeneca, Cytokinetics, Invitae Corporation, Janssen Pharmaceuticals, Pfizer Inc, PepGen Inc, Tenaya Therapeutics, and Stealth BioTherapeutics Inc; she is also the founder of Ikaika Therapeutics. E.E.K. received personal fees from Illumina Inc, 23andMe, and Regeneron Pharmaceuticals and serves as a scientific advisory board member for Encompass Bioscience, Foresite Labs, and Galateo Bio. B.K. is an advisory board member and stockholder of Genome Medical. M.S. is a member of the Institutional Review Board of the All of Us Research Program. E.F.P. is a paid consultant for Allecia Inc. J.F.P. is a paid consultant for Natera Inc. All other authors declare no conflicts of interest.
Figures


References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi:10.1161/CIR.0000000000000625 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- K12 AR084232/AR/NIAMS NIH HHS/United States
- U01 HG011167/HG/NHGRI NIH HHS/United States
- U01 HG011172/HG/NHGRI NIH HHS/United States
- U01 HG008657/HG/NHGRI NIH HHS/United States
- S10 OD026880/OD/NIH HHS/United States
- U01 HG011169/HG/NHGRI NIH HHS/United States
- R01 HL128075/HL/NHLBI NIH HHS/United States
- UL1 TR004419/TR/NCATS NIH HHS/United States
- K12 HD043483/HD/NICHD NIH HHS/United States
- U01 HG008685/HG/NHGRI NIH HHS/United States
- U01 HG011181/HG/NHGRI NIH HHS/United States
- U01 HG011175/HG/NHGRI NIH HHS/United States
- K24 HL133373/HL/NHLBI NIH HHS/United States
- S10 OD030463/OD/NIH HHS/United States
- U01 HG008680/HG/NHGRI NIH HHS/United States
- U01 HG011176/HG/NHGRI NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- U01 HG006379/HG/NHGRI NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- U01 HG011166/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

