Prognostic value of modified criteria for hydroxyurea resistance or intolerance in patients with high-risk essential thrombocythemia
- PMID: 36622093
- PMCID: PMC10134319
- DOI: 10.1002/cam4.5602
Prognostic value of modified criteria for hydroxyurea resistance or intolerance in patients with high-risk essential thrombocythemia
Abstract
Background: Recognizing intolerance or resistance to hydroxyurea (HU), which is related to the high risk of a disease transformation and reduced survival in essential thrombocythemia (ET), is crucial to making a reasonable decision about second-line therapy. We assessed the prognostic impact of the modified European LeukemiaNet (mELN) criteria used in a recent MAJIC-ET trial by analyzing the incidence of resistance or intolerance to HU and the survival outcome compared with those of the ELN criteria.
Methods: We retrospectively compared the development of HU resistance or intolerance according to the ELN and mELN criteria for 148 high-risk ET patients receiving HU between 2014 and 2018. The maximum tolerated dose for defining HU resistance was used in the mELN criteria.
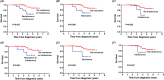
Results: The median age of patients was 65 years (range, 36-87), with a median follow-up of 3.6 years (range, 1.1-6.4). Two thromboembolic events were observed during HU treatment. When applying the ELN criteria, 10 patients (6.9%) were resistant (n = 5 [3.4%]) or intolerant (n = 5 [3.4%]) to HU in comparison with 22 patients (15%, 14 [9.8%]) resistant and 8 [5.5%] intolerant when applying the mELN criteria. Transformation to myelofibrosis and acute myeloid leukemia occurred in 2 (1.4%) patients and 1 (0.7%) patient, respectively, as defined by the ELN criteria compared with 3 (2.1%) and 2 (1.4%) patients as defined by the mELN criteria. In multivariate analysis of transformation-free survival, HU resistance defined by the mELN criteria but not the ELN criteria was an independent prognostic factor. In addition, HU resistance as defined by both sets of criteria was an independent risk factor for inferior overall survival. Intolerance of HU did not have any prognostic impact on survival.
Conclusions: The mELN criteria are useful for identifying high-risk ET patients who might be eligible for second-line therapy in practice, which should be validated in a prospective setting.
Keywords: essential thrombocythemia; hydroxyurea; intolerance; resistance.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors made no disclosures.
Figures

References
-
- Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk‐stratification and management. Am J Hematol. 2019;94:133‐143. - PubMed
-
- Tefferi A, Pardanani A. Essential thrombocythemia. N Engl J Med. 2019;381:2135‐2144. - PubMed
-
- Podoltsev NA, Zhu M, Zeidan AM, et al. Impact of hydroxyurea on survival and risk of thrombosis among older patients with essential thrombocythemia. J Natl Compr Canc Netw. 2019;17:211‐219. - PubMed
-
- Rumi E, Cazzola M. How I treat essential thrombocythemia. Blood. 2016;128:2403‐2414. - PubMed
-
- Besses C, Alvarez‐Larran A. How to treat essential thrombocythemia and polycythemia vera. Clin Lymphoma Myeloma Leuk. 2016;16(Suppl):S114‐S123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

