Influence of Hormonal Contraceptive Use and HIV on Cervicovaginal Cytokines and Microbiota in Malawi
- PMID: 36622252
- PMCID: PMC9942570
- DOI: 10.1128/msphere.00585-22
Influence of Hormonal Contraceptive Use and HIV on Cervicovaginal Cytokines and Microbiota in Malawi
Abstract
Important questions remain on how hormonal contraceptives alter the local immune environment and the microbiota in the female genital tract and how such effects may impact susceptibility to HIV infection. We leveraged samples from a previously conducted clinical trial of Malawian women with (n = 73) and without (n = 24) HIV infection randomized to depot medroxyprogesterone acetate (DMPA) or the levonogestrel implant in equal numbers within each group and determined the effects of these hormonal contraceptives (HCs) on the vaginal immune milieu and the composition of the vaginal microbiota. Longitudinal data for soluble immune mediators, measured by multiplex bead arrays and enzyme-linked immunosorbent assays (ELISAs), and vaginal microbiota, assessed by 16S rRNA gene amplicon, were collected prior to and over a period of 180 days post-HC initiation. DMPA and levonogestrel had only minimal effects on the vaginal immune milieu and microbiota. In women with HIV, with the caveat of a small sample size, there was an association between the median log10 change in the interleukin-12 (IL-12)/IL-10 ratio in vaginal fluid at day 180 post-HC compared to baseline when these women were classified as having a community state type (CST) IV vaginal microbiota and were randomized to DMPA. Long-lasting alterations in soluble immune markers or shifts in microbiota composition were not observed. Furthermore, women with HIV did not exhibit increased viral shedding in the genital tract after HC initiation. Consistent with the results of the ECHO (Evidence for Contraceptive Options and HIV Outcomes) trial, our data imply that the progestin-based HC DMPA and levonorgestrel are associated with minimal risk for women with HIV. (This study has been registered at ClinicalTrials.gov under registration no. NCT02103660). IMPORTANCE The results of the Evidence for Contraceptive Options and HIV Outcomes (ECHO) trial, the first large randomized controlled clinical trial comparing the HIV acquisition risk of women receiving DMPA, the levonorgestrel (LNG) implant, or the copper intrauterine device (IUD), did not reveal an increased risk of HIV acquisition for women on any of these three contraceptives. Our study results confirm that the two different progestin-based hormonal contraceptives DMPA and levonogestrel will not increase the risk for HIV infection. Furthermore, DMPA and levonogestrel have only minimal effects on the immune milieu and the microbiota in the vaginal tract, attesting to the safety of these hormonal contraceptives.
Keywords: HIV; contraception; cytokines; depot medroxyprogesterone acetate injectable; inflammation; levonorgestrel implant; microbiome; progestin; progestin contraception.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
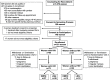


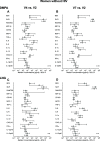


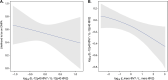
References
-
- Morrison CS, Chen PL, Kwok C, Baeten JM, Brown J, Crook AM, Van Damme L, Delany-Moretlwe S, Francis SC, Friedland BA, Hayes RJ, Heffron R, Kapiga S, Karim QA, Karpoff S, Kaul R, McClelland RS, McCormack S, McGrath N, Myer L, Rees H, van der Straten A, Watson-Jones D, van de Wijgert JH, Stalter R, Low N. 2015. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med 12:e1001778. doi:10.1371/journal.pmed.1001778. - DOI - PMC - PubMed
-
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Consortium. 2019. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 394:303–313. doi:10.1016/S0140-6736(19)31288-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- OPP1090837/Bill and Melinda Gates Foundation (GF)
- U48DP00194/HHS | Centers for Disease Control and Prevention (CDC)
- 200-2015-M-63021/HHS | Centers for Disease Control and Prevention (CDC)
- P30-AI50410/HHS | NIH | Office of Extramural Research, National Institutes of Health (OER)
- 1K01-TW009657-01/HHS | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical

