SLAMF6 compartmentalization enhances T cell functions
- PMID: 36622343
- PMCID: PMC9733572
- DOI: 10.26508/lsa.202201533
SLAMF6 compartmentalization enhances T cell functions
Abstract
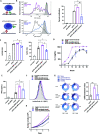
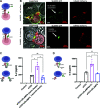
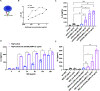
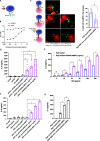
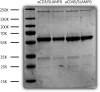
Signaling lymphocyte activation molecule family member 6 (SLAMF6) is a T cell co-receptor. Previously, we showed that SLAMF6 clustering was required for T cell activation. To better understand the relationship between SLAMF6 location and function and to evaluate the role of SLAMF6 as a therapeutic target, we investigated how its compartmentalization on the cell surface affects T cell functions. We used biochemical and co-culture assays to show that T cell activity is enhanced when SLAMF6 colocalizes with the CD3 complex. Mechanistically, co-immunoprecipitation analysis revealed the SLAMF6-interacting proteins to be those essential for signaling downstream of T cell receptor, suggesting the two receptors share downstream signaling pathways. Bispecific anti-CD3/SLAMF6 antibodies, designed to promote SLAMF6 clustering with CD3, enhanced T cell activation. Meanwhile, anti-CD45/SLAMF6 antibodies inhibited SLAMF6 clustering with T cell receptor, likely because of the steric hindrance, but nevertheless enhanced T cell activation. We conclude that SLAMF6 bispecific antibodies have a role in modulating T cell responses, and future work will evaluate the therapeutic potential in tumor models.
© 2022 Gartshteyn et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Eisenberg G, Engelstein R, Geiger-Maor A, Hajaj E, Merims S, Frankenburg S, Uzana R, Rutenberg A, Machlenkin A, Frei G, et al. (2018) Soluble SLAMF6 receptor induces strong CD8(+) T-cell effector function and improves anti-melanoma activity in vivo. Cancer Immunol Res 6: 127–138. 10.1158/2326-6066.cir-17-0383 - DOI - PubMed
-
- Schenkel JM, Herbst RH, Canner D, Li A, Hillman M, Shanahan SL, Gibbons G, Smith OC, Kim JY, Westcott P, et al. (2021) Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1(+) CD8(+) T cells in tumor-draining lymph nodes. Immunity 54: 2338–2353.e6. 10.1016/j.immuni.2021.08.026 - DOI - PMC - PubMed
-
- Hajaj E, Eisenberg G, Klein S, Frankenburg S, Merims S, Ben David I, Eisenhaure T, Henrickson SE, Villani AC, Hacohen N, et al. (2020) SLAMF6 deficiency augments tumor killing and skews toward an effector phenotype revealing it as a novel T cell checkpoint. Elife 9: e52539. 10.7554/elife.52539 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
