Assessment of Safety of a Fully Implanted Endovascular Brain-Computer Interface for Severe Paralysis in 4 Patients: The Stentrode With Thought-Controlled Digital Switch (SWITCH) Study
- PMID: 36622685
- PMCID: PMC9857731
- DOI: 10.1001/jamaneurol.2022.4847
Assessment of Safety of a Fully Implanted Endovascular Brain-Computer Interface for Severe Paralysis in 4 Patients: The Stentrode With Thought-Controlled Digital Switch (SWITCH) Study
Erratum in
-
Error in Figure 3.JAMA Neurol. 2023 May 1;80(5):533. doi: 10.1001/jamaneurol.2023.0594. JAMA Neurol. 2023. PMID: 36972039 Free PMC article. No abstract available.
Abstract
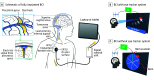
Importance: Brain-computer interface (BCI) implants have previously required craniotomy to deliver penetrating or surface electrodes to the brain. Whether a minimally invasive endovascular technique to deliver recording electrodes through the jugular vein to superior sagittal sinus is safe and feasible is unknown.
Objective: To assess the safety of an endovascular BCI and feasibility of using the system to control a computer by thought.
Design, setting, and participants: The Stentrode With Thought-Controlled Digital Switch (SWITCH) study, a single-center, prospective, first in-human study, evaluated 5 patients with severe bilateral upper-limb paralysis, with a follow-up of 12 months. From a referred sample, 4 patients with amyotrophic lateral sclerosis and 1 with primary lateral sclerosis met inclusion criteria and were enrolled in the study. Surgical procedures and follow-up visits were performed at the Royal Melbourne Hospital, Parkville, Australia. Training sessions were performed at patients' homes and at a university clinic. The study start date was May 27, 2019, and final follow-up was completed January 9, 2022.
Interventions: Recording devices were delivered via catheter and connected to subcutaneous electronic units. Devices communicated wirelessly to an external device for personal computer control.
Main outcomes and measures: The primary safety end point was device-related serious adverse events resulting in death or permanent increased disability. Secondary end points were blood vessel occlusion and device migration. Exploratory end points were signal fidelity and stability over 12 months, number of distinct commands created by neuronal activity, and use of system for digital device control.
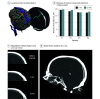
Results: Of 4 patients included in analyses, all were male, and the mean (SD) age was 61 (17) years. Patients with preserved motor cortex activity and suitable venous anatomy were implanted. Each completed 12-month follow-up with no serious adverse events and no vessel occlusion or device migration. Mean (SD) signal bandwidth was 233 (16) Hz and was stable throughout study in all 4 patients (SD range across all sessions, 7-32 Hz). At least 5 attempted movement types were decoded offline, and each patient successfully controlled a computer with the BCI.
Conclusions and relevance: Endovascular access to the sensorimotor cortex is an alternative to placing BCI electrodes in or on the dura by open-brain surgery. These final safety and feasibility data from the first in-human SWITCH study indicate that it is possible to record neural signals from a blood vessel. The favorable safety profile could promote wider and more rapid translation of BCI to people with paralysis.
Trial registration: ClinicalTrials.gov Identifier: NCT03834857.
Conflict of interest statement
Figures

References
-
- McKinsey Global Institute . Disruptive technologies: advances that will transform life, business, and the global economy. Accessed September 6, 2022. https://www.mckinsey.com/~/media/mckinsey/business%20functions/mckinsey%...
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

