Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy
- PMID: 36622892
- PMCID: PMC10283420
- DOI: 10.1080/15548627.2023.2165313
Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy
Abstract
Microglial phagocytosis of apoptotic debris prevents buildup damage of neighbor neurons and inflammatory responses. Whereas microglia are very competent phagocytes under physiological conditions, we report their dysfunction in mouse and preclinical monkey models of stroke (macaques and marmosets) by transient occlusion of the medial cerebral artery (tMCAo). By analyzing recently published bulk and single cell RNA sequencing databases, we show that the phagocytosis dysfunction was not explained by transcriptional changes. In contrast, we demonstrate that the impairment of both engulfment and degradation was related to energy depletion triggered by oxygen and nutrient deprivation (OND), which led to reduced process motility, lysosomal exhaustion, and the induction of a protective macroautophagy/autophagy response in microglia. Basal autophagy, in charge of removing and recycling intracellular elements, was critical to maintain microglial physiology, including survival and phagocytosis, as we determined both in vivo and in vitro using pharmacological and transgenic approaches. Notably, the autophagy inducer rapamycin partially prevented the phagocytosis impairment induced by tMCAo in vivo but not by OND in vitro, where it even had a detrimental effect on microglia, suggesting that modulating microglial autophagy to optimal levels may be a hard to achieve goal. Nonetheless, our results show that pharmacological interventions, acting directly on microglia or indirectly on the brain environment, have the potential to recover phagocytosis efficiency in the diseased brain. We propose that phagocytosis is a therapeutic target yet to be explored in stroke and other brain disorders and provide evidence that it can be modulated in vivo using rapamycin.Abbreviations: AIF1/IBA1: allograft inflammatory factor 1; AMBRA1: autophagy/beclin 1 regulator 1; ATG4B: autophagy related 4B, cysteine peptidase; ATP: adenosine triphosphate; BECN1: beclin 1, autophagy related; CASP3: caspase 3; CBF: cerebral blood flow; CCA: common carotid artery; CCR2: chemokine (C-C motif) receptor 2; CIR: cranial irradiation; Csf1r/v-fms: colony stimulating factor 1 receptor; CX3CR1: chemokine (C-X3-C motif) receptor 1; DAPI: 4',6-diamidino-2-phenylindole; DG: dentate gyrus; GO: Gene Ontology; HBSS: Hanks' balanced salt solution; HI: hypoxia-ischemia; LAMP1: lysosomal-associated membrane protein 1; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MCA: medial cerebral artery; MTOR: mechanistic target of rapamycin kinase; OND: oxygen and nutrient deprivation; Ph/A coupling: phagocytosis-apoptosis coupling; Ph capacity: phagocytic capacity; Ph index: phagocytic index; SQSTM1: sequestosome 1; RNA-Seq: RNA sequencing; TEM: transmission electron microscopy; tMCAo: transient medial cerebral artery occlusion; ULK1: unc-51 like kinase 1.
Keywords: Autophagy; ischemia; lysosomes; microglia; phagocytosis; rapamycin; stroke; tMCAo.
Conflict of interest statement
Authors declare no competing financial interests.
Figures
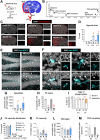
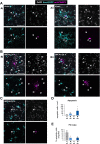




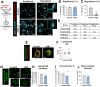



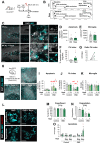

References
-
- Ma Y, Wang J, Wang Y, et al. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017. Oct;157:247–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
