Increased Proportion of Colorectal Cancer in Patients With Ulcerative Colitis Undergoing Surgery in the Netherlands
- PMID: 36623170
- PMCID: PMC10144357
- DOI: 10.14309/ajg.0000000000002099
Increased Proportion of Colorectal Cancer in Patients With Ulcerative Colitis Undergoing Surgery in the Netherlands
Abstract
Introduction: The aim of the current study was to assess whether there is an indication shift for surgery in patients with ulcerative colitis (UC) from refractory disease to malignant degeneration over the past 3 decades.
Methods: All patients with histologically confirmed UC who underwent a colorectal resection between 1991 and 2020 were extracted from the nationwide Dutch Pathology Registry. The primary outcome was the proportion of colorectal cancer (CRC) in the colon specimens. Outcomes were compared between 3 periods (P1: 1991-2000, P2: 2001-2010, and P3: 2011-2020).
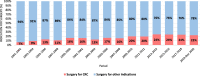
Results: Overall, 6,094 patients with UC were included of which 4,854 underwent a (procto)colectomy and 1,240 a segmental resection. In 1,031 (16.9%) patients, CRC was demonstrated in the pathological resection specimen after a median disease duration of 11 years (IQR 3.0-19.0). The proportion of CRC increased from 11.3% in P1, to 16.1% in P2, and 22.8% in P3 ( P < 0.001). Median disease duration at the time of resection increased from 4 years in P1, to 10 years in P2, and 17 years in P3 ( P < 0.001). The proportion of patients diagnosed with advanced malignancy (pT3/T4) (P1: 61.2% vs P2: 65.2% vs P3: 62.4%, respectively, P = 0.633) and lymph node metastasis (N+) (P1: 33.0% vs P2: 41.9% vs P3: 38.2%, respectively, P = 0.113) did not change over time.
Discussion: This nationwide pathology study demonstrated an increased proportion of surgery for CRC over the past 3 decades. We hypothesize that the expanding therapeutic armamentarium for UC leads to exhausting medical options and hence postponed colectomy. This, however, might be at the expense of an increased risk of CRC in the long term.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures
References
-
- Jess T, Rungoe C, Peyrin–Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol 2012;10(6):639–45. - PubMed
-
- Lutgens MWMD, van Oijen MGH, van der Heijden GJMG, et al. . Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013;19(4):789–99. - PubMed
-
- Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: The declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther 2014;39(7):645–59. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous