High-Dose Once-Daily Thoracic Radiotherapy in Limited-Stage Small-Cell Lung Cancer: CALGB 30610 (Alliance)/RTOG 0538
- PMID: 36623230
- PMCID: PMC10150922
- DOI: 10.1200/JCO.22.01359
High-Dose Once-Daily Thoracic Radiotherapy in Limited-Stage Small-Cell Lung Cancer: CALGB 30610 (Alliance)/RTOG 0538
Abstract
Purpose: Although level 1 evidence supports 45-Gy twice-daily radiotherapy as standard for limited-stage small-cell lung cancer, most patients receive higher-dose once-daily regimens in clinical practice. Whether increasing radiotherapy dose improves outcomes remains to be prospectively demonstrated.
Methods: This phase III trial, CALGB 30610/RTOG 0538 (ClinicalTrials.gov identifier: NCT00632853), was conducted in two stages. In the first stage, patients with limited-stage disease were randomly assigned to receive 45-Gy twice-daily, 70-Gy once-daily, or 61.2-Gy concomitant-boost radiotherapy, starting with either the first or second (of four total) chemotherapy cycles. In the second stage, allocation to the 61.2-Gy arm was discontinued following planned interim toxicity analysis, and the study continued with two remaining arms. The primary end point was overall survival (OS) in the intention-to-treat population.
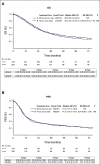
Results: Trial accrual opened on March 15, 2008, and closed on December 1, 2019. All patients randomly assigned to 45-Gy twice-daily (n = 313) or 70-Gy once-daily radiotherapy (n = 325) are included in this analysis. After a median follow-up of 4.7 years, OS was not improved on the once-daily arm (hazard ratio for death, 0.94; 95% CI, 0.76 to 1.17; P = .594). Median survival is 28.5 months for twice-daily treatment, and 30.1 months for once-daily treatment, with 5-year OS of 29% and 32%, respectively. Treatment was tolerable, and the frequency of severe adverse events, including esophageal and pulmonary toxicity, was similar on both arms.
Conclusion: Although 45-Gy twice-daily radiotherapy remains the standard of care, this study provides the most robust information available to help guide the choice of thoracic radiotherapy regimen for patients with limited-stage small-cell lung cancer.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Govindan R, Page N, Morgensztern D, et al. : Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539-4544, 2006 - PubMed
-
- Zelen M: Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 4:31-42, 1973 - PubMed
-
- Turrisi AT III, Kim K, Blum R, et al. : Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265-271, 1999 - PubMed
-
- Farrell MJ, Yahya JB, Degnin C, et al. : Radiation dose and fractionation for limited-stage small-cell lung cancer: Survey of US radiation oncologists on practice patterns. Clin Lung Cancer 20:13-19, 2019 - PubMed
-
- Bogart JA, Herndon JE II, Lyss AP, et al. : 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: Analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys 59:460-468, 2004 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical