Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors
- PMID: 36623241
- PMCID: PMC10489404
- DOI: 10.1200/JCO.22.01351
Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors
Abstract
Purpose: Pembrolizumab significantly improves clinical outcomes in advanced/metastatic microsatellite instability high (MSI-H)/deficient mismatch repair (dMMR) solid tumors but is not well studied in the neoadjuvant space.
Methods: This is a phase II open-label, single-center trial of localized unresectable or high-risk resectable MSI-H/dMMR tumors. Treatment is pembrolizumab 200 mg once every 3 weeks for 6 months followed by surgical resection with an option to continue therapy for 1 year followed by observation. To continue on study, patients are required to have radiographic or clinical benefit. The coprimary end points are safety and pathologic complete response. Key secondary end points are response rate and organ-sparing at one year for patients who declined surgery. Exploratory analyses include interrogation of the tumor immune microenvironment using imaging mass cytometry.
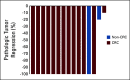
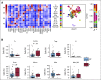
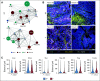
Results: A total of 35 patients were enrolled, including 27 patients with colorectal cancer and eight patients with noncolorectal cancer. Among 33 evaluable patients, best overall response rate was 82%. Among 17 (49%) patients who underwent surgery, the pathologic complete response rate was 65%. Ten patients elected to receive one year of pembrolizumab followed by surveillance without surgical resection (median follow-up of 23 weeks [range, 0-54 weeks]). An additional eight did not undergo surgical resection and received less than 1 year of pembrolizumab. During the study course of the trial and subsequent follow-up, progression events were seen in six patients (four of whom underwent salvage surgery). There were no new safety signals. Spatial immune profiling with imaging mass cytometry noted a significantly closer proximity between granulocytic cells and cytotoxic T cells in patients with progressive events compared with those without progression.
Conclusion: Neoadjuvant pembrolizumab in dMMR/MSI-H cancers is safe and resulted in high rates of pathologic, radiographic, and endoscopic response, which has implications for organ-sparing strategies.
Trial registration: ClinicalTrials.gov NCT04082572.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

Comment in
-
Immunotherapy in Localized Microsatellite Instability-High/Mismatch Repair Deficient Solid Tumors: Are We Ready for a New Standard of Care?J Clin Oncol. 2023 Apr 20;41(12):2138-2140. doi: 10.1200/JCO.22.02564. Epub 2023 Jan 9. J Clin Oncol. 2023. PMID: 36623236 No abstract available.
-
Neoadjuvant pembrolizumab shows promise in MSI-H/dMMR solid tumours.Nat Rev Clin Oncol. 2023 Mar;20(3):138. doi: 10.1038/s41571-023-00732-7. Nat Rev Clin Oncol. 2023. PMID: 36650361 No abstract available.
References
-
- Germano G, Amirouchene-Angelozzi N, Rospo G, et al. : The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov 8:1518-1528, 2018 - PubMed
-
- Brueckl WM, Moesch C, Brabletz T, et al. : Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res 23:1773-1777, 2003 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

