Advancing Survivors Knowledge (ASK Study) of Skin Cancer Surveillance After Childhood Cancer: A Randomized Controlled Trial in the Childhood Cancer Survivor Study
- PMID: 36623247
- PMCID: PMC10448942
- DOI: 10.1200/JCO.22.00408
Advancing Survivors Knowledge (ASK Study) of Skin Cancer Surveillance After Childhood Cancer: A Randomized Controlled Trial in the Childhood Cancer Survivor Study
Abstract
Purpose: To improve skin cancer screening among survivors of childhood cancer treated with radiotherapy where skin cancers make up 58% of all subsequent neoplasms. Less than 30% of survivors currently complete recommended skin cancer screening.
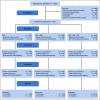
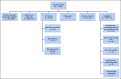
Patients and methods: This randomized controlled comparative effectiveness trial evaluated patient and provider activation (PAE + MD) and patient and provider activation with teledermoscopy (PAE + MD + TD) compared with patient activation alone (PAE), which included print materials, text messaging, and a website on skin cancer risk factors and screening behaviors. Seven hundred twenty-eight participants from the Childhood Cancer Survivor Study (median age at baseline 44 years), age > 18 years, treated with radiotherapy as children, and without previous history of skin cancer were randomly assigned (1:1:1). Primary outcomes included receiving a physician skin examination at 12 months and conducting a skin self-examination at 18 months after intervention.
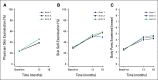
Results: Rates of physician skin examinations increased significantly from baseline to 12 months in all three intervention groups: PAE, 24%-39%, relative risk [RR], 1.65, 95% CI, 1.32 to 2.08; PAE + MD, 24% to 39%, RR, 1.56, 95% CI, 1.25 to 1.97; PAE + MD + TD, 24% to 46%, RR, 1.89, 95% CI, 1.51 to 2.37. The increase in rates did not differ between groups (P = .49). Similarly, rates of skin self-examinations increased significantly from baseline to 18 months in all three groups: PAE, 29% to 50%, RR, 1.75, 95% CI, 1.42 to 2.16; PAE + MD, 31% to 58%, RR, 1.85, 95% CI, 1.52 to 2.26; PAE + MD + TD, 29% to 58%, RR, 1.95, 95% CI, 1.59 to 2.40, but the increase in rates did not differ between groups (P = .43).
Conclusion: Although skin cancer screening rates increased more than 1.5-fold in each of the intervention groups, there were no differences between groups. Any of these interventions, if implemented, could improve skin cancer prevention behaviors among childhood cancer survivors.
Trial registration: ClinicalTrials.gov NCT02046811.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2018. National Cancer Institute; 2021. https://seer.cancer.gov/csr/1975_2018/
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

