Risks and burdens of incident dyslipidaemia in long COVID: a cohort study
- PMID: 36623520
- PMCID: PMC9873268
- DOI: 10.1016/S2213-8587(22)00355-2
Risks and burdens of incident dyslipidaemia in long COVID: a cohort study
Abstract
Background: Non-clinical evidence and a few human studies with short follow-ups suggest increased risk of dyslipidaemia in the post-acute phase of COVID-19 (ie, >30 days after SARS-CoV-2 infection). However, detailed large-scale controlled studies with longer follow-ups and in-depth assessment of the risks and burdens of incident dyslipidaemia in the post-acute phase of COVID-19 are not yet available. We, therefore, aimed to examine the risks and 1-year burdens of incident dyslipidaemia in the post-acute phase of COVID-19 among people who survive the first 30 days of SARS-CoV-2 infection.
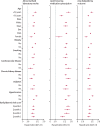
Methods: In this cohort study, we used the national health-care databases of the US Department of Veterans Affairs to build a cohort of 51 919 participants who had a positive COVID-19 test and survived the first 30 days of infection between March 1, 2020, and Jan 15, 2021; a non-infected contemporary control group (n=2 647 654) that enrolled patients between March 1, 2020, and Jan 15, 2021; and a historical control group (n=2 539 941) that enrolled patients between March 1, 2018, and Jan 15, 2019. Control groups had no evidence of SARS-CoV-2 infection, and participants in all three cohorts were free of dyslipidaemia before cohort enrolment. We then used inverse probability weighting using predefined and algorithmically-selected high dimensional variables to estimate the risks and 1-year burdens of incident dyslipidaemia, lipid-lowering medications use, and a composite of these outcomes. We reported two measures of risk: hazard ratios (HRs) and burden per 1000 people at 12 months. Additionally, we estimated the risks and burdens of incident dyslipidaemia outcomes in mutually exclusive groups based on the care setting of the acute infection (ie, participants who were non-hospitalised, hospitalised, or admitted to intensive care during the acute phase of SARS-CoV-2 infection).
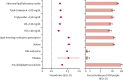
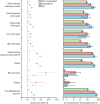
Findings: In the post-acute phase of the SARS-CoV-2 infection, compared with the non-infected contemporary control group, those in the COVID-19 group had higher risks and burdens of incident dyslipidaemia, including total cholesterol greater than 200 mg/dL (hazard ratio [HR] 1·26, 95% CI 1·22-1·29; burden 22·46, 95% CI 19·14-25·87 per 1000 people at 1 year), triglycerides greater than 150 mg/dL (1·27, 1·23-1·31; 22·03, 18·85-25·30), LDL cholesterol greater than 130 mg/dL (1·24, 1·20-1·29; 18·00, 14·98-21·11), and HDL cholesterol lower than 40 mg/dL (1·20, 1·16-1·25; 15·58, 12·52-18·73). The risk and burden of a composite of these abnormal lipid laboratory outcomes were 1·24 (95% CI 1·21-1·27) and 39·19 (95% CI 34·71-43·73), respectively. There was also increased risk and burden of incident lipid-lowering medications use (HR 1·54, 95% CI 1·48-1·61; burden 25·50, 95% CI 22·61-28·50). A composite of any dyslipidaemia outcome (laboratory abnormality or lipid-lowering medications use) yielded an HR of 1·31 (95% CI 1·28-1·34) and a burden of 54·03 (95% CI 49·21-58·92). The risks and burdens of these post-acute outcomes increased in a graded fashion corresponding to the severity of the acute phase of COVID-19 infection (ie, whether patients were non-hospitalised, hospitalised, or admitted to intensive care). The results were consistent in analyses comparing the COVID-19 group to the non-infected historical control group.
Interpretation: Our findings suggest increased risks and 1-year burdens of incident dyslipidaemia and incident lipid-lowering medications use in the post-acute phase of COVID-19 infection. Post-acute care for those with COVID-19 should involve attention to dyslipidaemia as a potential post-acute sequela of SARS-CoV-2 infection.
Funding: US Department of Veterans Affairs.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Blood lipids after COVID-19 infection.Lancet Diabetes Endocrinol. 2023 Feb;11(2):68-69. doi: 10.1016/S2213-8587(22)00389-8. Epub 2023 Jan 6. Lancet Diabetes Endocrinol. 2023. PMID: 36623516 Free PMC article. No abstract available.
Similar articles
-
Risks and burdens of incident diabetes in long COVID: a cohort study.Lancet Diabetes Endocrinol. 2022 May;10(5):311-321. doi: 10.1016/S2213-8587(22)00044-4. Epub 2022 Mar 21. Lancet Diabetes Endocrinol. 2022. PMID: 35325624 Free PMC article.
-
Risks of mental health outcomes in people with covid-19: cohort study.BMJ. 2022 Feb 16;376:e068993. doi: 10.1136/bmj-2021-068993. BMJ. 2022. PMID: 35172971 Free PMC article.
-
Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study.Lancet Infect Dis. 2024 Mar;24(3):239-255. doi: 10.1016/S1473-3099(23)00684-9. Epub 2023 Dec 14. Lancet Infect Dis. 2024. PMID: 38104583
-
Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: Multi-sourced population-based health records cohort study.Thromb Res. 2021 Jun;202:17-23. doi: 10.1016/j.thromres.2021.03.006. Epub 2021 Mar 8. Thromb Res. 2021. PMID: 33711754 Free PMC article.
-
New-onset type 1 diabetes in children and adolescents as postacute sequelae of SARS-CoV-2 infection: A systematic review and meta-analysis of cohort studies.J Med Virol. 2023 Jun;95(6):e28833. doi: 10.1002/jmv.28833. J Med Virol. 2023. PMID: 37264687
Cited by
-
The Significance of Endothelial Dysfunction in Long COVID-19 for the Possible Future Pandemic of Chronic Kidney Disease and Cardiovascular Disease.Biomolecules. 2024 Aug 8;14(8):965. doi: 10.3390/biom14080965. Biomolecules. 2024. PMID: 39199353 Free PMC article. Review.
-
Association of systemic immune-inflammation index with diabetic kidney disease in patients with type 2 diabetes: a cross-sectional study in Chinese population.Front Endocrinol (Lausanne). 2024 Jan 4;14:1307692. doi: 10.3389/fendo.2023.1307692. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38239983 Free PMC article.
-
The Intersection Between COVID-19, Cardiovascular Disease, and Diet: a Review.Curr Atheroscler Rep. 2023 Oct;25(10):643-652. doi: 10.1007/s11883-023-01138-7. Epub 2023 Aug 30. Curr Atheroscler Rep. 2023. PMID: 37646976 Review.
-
The plasma metabolome of long COVID patients two years after infection.Sci Rep. 2023 Aug 1;13(1):12420. doi: 10.1038/s41598-023-39049-x. Sci Rep. 2023. PMID: 37528111 Free PMC article.
-
Targeting the High-Density Lipoprotein Proteome for the Treatment of Post-Acute Sequelae of SARS-CoV-2.Int J Mol Sci. 2024 Apr 20;25(8):4522. doi: 10.3390/ijms25084522. Int J Mol Sci. 2024. PMID: 38674105 Free PMC article.
References
-
- Roccaforte V, Daves M, Lippi G, Spreafico M, Bonato C. Altered lipid profile in patients with COVID-19 infection. J Lab Precis Med. 2021;6:2.
-
- Chen Y, Yao H, Zhang N, et al. Proteomic analysis identifies prolonged disturbances in pathways related to cholesterol metabolism and myocardium function in the COVID-19 recovery stage. J Proteome Res. 2021;20:3463–3474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

