We Know What You Agreed To, Don't We?-Evaluating the Quality of Paper-Based Consents Forms and Their Digitalized Equivalent Using the Example of the Baltic Fracture Competence Centre Project
- PMID: 36623832
- PMCID: PMC10306442
- DOI: 10.1055/s-0042-1760249
We Know What You Agreed To, Don't We?-Evaluating the Quality of Paper-Based Consents Forms and Their Digitalized Equivalent Using the Example of the Baltic Fracture Competence Centre Project
Abstract
Introduction: The informed consent is the legal basis for research with human subjects. Therefore, the consent form (CF) as legally binding document must be valid, that is, be completely filled-in stating the person's decision clearly and signed by the respective person. However, especially paper-based CFs might have quality issues and the transformation into machine-readable information could add to low quality. This paper evaluates the quality and arising quality issues of paper-based CFs using the example of the Baltic Fracture Competence Centre (BFCC) fracture registry. It also evaluates the impact of quality assurance (QA) measures including giving site-specific feedback. Finally, it answers the question whether manual data entry of patients' decisions by clinical staff leads to a significant error rate in digitalized paper-based CFs.
Methods: Based on defined quality criteria, monthly QA including source data verification was conducted by two individual reviewers since the start of recruitment in December 2017. Basis for the analyses are the CFs collected from December 2017 until February 2019 (first recruitment period).
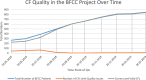
Results: After conducting QA internally, the sudden increase of quality issues in May 2018 led to site-specific feedback reports and follow-up training regarding the CFs' quality starting in June 2018. Specific criteria and descriptions on how to correct the CFs helped in increasing the quality in a timely matter. Most common issues were missing pages, decisions regarding optional modules, and signature(s). Since patients' datasets without valid CFs must be deleted, QA helped in retaining 65 datasets for research so that the final datapool consisted of 840 (99.29%) patients.
Conclusion: All quality issues could be assigned to one predefined criterion. Using the example of the BFCC fracture registry, CF-QA proved to significantly increase CF quality and help retain the number of available datasets for research. Consequently, the described quality indicators, criteria, and QA processes can be seen as the best practice approach.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
None declared.
Figures
References
-
- European Parliament, Council of the European Union . Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) Official J of the Eur Union L 119. 2016:1–88.
-
- World Medicine Association . Fortaleza, Brazil: WMA General Assembly; 2013. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects.
-
- Vogele D, Schöffski O, Efinger K, Schmidt S A, Beer M, Kildal D. Analysis of documented informed consent forms for computed tomography: completeness and data quality in four clinics. Radiologe. 2020;60(02):162–168. - PubMed
-
- BFCC project. Transnational Fracture Registry Platform. Published 2022. Accessed June 28, 2022 at:https://www.bfcc-project.eu/registry.html
-
- Pommerening K, Drepper J, Helbing K, Ganslandt T.Leitfaden zum Datenschutz in medizinischen Forschungsprojekten - Generische Lösungen der TMF 2.0. Vol Bd11:Medizinisch Wissenschaftliche Verlagsgesellschaft; 2014