Zoledronic acid targets chemo-resistant polyploid giant cancer cells
- PMID: 36624105
- PMCID: PMC9829701
- DOI: 10.1038/s41598-022-27090-1
Zoledronic acid targets chemo-resistant polyploid giant cancer cells
Abstract
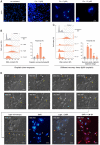
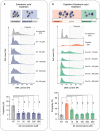
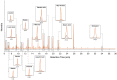
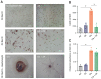
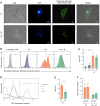
Although polyploid giant cancer cells (PGCCs) are known as a key source of failure of current therapies, sufficient drugs to target these cells are not yet introduced. Considering the similarities of polyploid cells in regeneration and cancer, we hypothesized that zoledronic acid (ZA), an osteoclast-targeting agent, might be used to eliminate PGCCs. The 5637-bladder cancer cell line was treated with various doses of cisplatin to enrich polyploid cells and the efficacy of different concentrations of ZA in reducing this population was assessed. The metabolic profile of PGCCs was investigated with gas chromatography-mass spectrometry. Lipid profiles, mitochondrial density, and ROS content were also measured to assess the response of the cells to ZA. Cancer cells surviving after three days of exposure with 6 μM cisplatin were mainly polyploid. These cells demonstrated special morphological features such as fusion with diploid or other polyploid cells and originated in daughter cells through budding. ZA could substantially eradicate PGCCs with the maximal effect observed with 50 μM which resulted in the drop of PGCC fraction from 60 ± 7.5 to 19 ± 1.7%. Enriched PGCCs after cisplatin-treatment demonstrated a drastic metabolic shift compared to untreated cancer cells with an augmentation of lipids. Further assays confirmed the high content of lipid droplets and cholesterol in these cells which were reduced after ZA administration. Additionally, the mitochondrial density and ROS increased in PGCCs both of which declined in response to ZA. Taken together, we propose that ZA is a potent inhibitor of PGCCs which alters the metabolism of PGCCs. Although this drug has been successfully exploited as adjuvant therapy for some malignancies, the current evidence on its effects on PGCCs justifies further trials to assess its potency for improving the success of current therapies for tackling tumor resistance and relapse.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Dormant cancer cells and polyploid giant cancer cells: The roots of cancer recurrence and metastasis.Clin Transl Med. 2024 Feb;14(2):e1567. doi: 10.1002/ctm2.1567. Clin Transl Med. 2024. PMID: 38362620 Free PMC article. Review.
-
Autophagy modulating therapeutics inhibit ovarian cancer colony generation by polyploid giant cancer cells (PGCCs).BMC Cancer. 2022 Apr 14;22(1):410. doi: 10.1186/s12885-022-09503-6. BMC Cancer. 2022. PMID: 35421971 Free PMC article.
-
Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells.Oncogene. 2017 Aug 24;36(34):4887-4900. doi: 10.1038/onc.2017.72. Epub 2017 Apr 24. Oncogene. 2017. PMID: 28436947 Free PMC article.
-
A Fungicide, Fludioxonil, Formed the Polyploid Giant Cancer Cells and Induced Metastasis and Stemness in MDA-MB-231 Triple-Negative Breast Cancer Cells.Int J Mol Sci. 2024 Aug 20;25(16):9024. doi: 10.3390/ijms25169024. Int J Mol Sci. 2024. PMID: 39201710 Free PMC article.
-
Polyploid giant cancer cell characterization: New frontiers in predicting response to chemotherapy in breast cancer.Semin Cancer Biol. 2022 Jun;81:220-231. doi: 10.1016/j.semcancer.2021.03.017. Epub 2021 Mar 22. Semin Cancer Biol. 2022. PMID: 33766651 Free PMC article. Review.
Cited by
-
High-Throughput Empirical and Virtual Screening to Discover Novel Inhibitors of Polyploid Giant Cancer Cells in Breast Cancer.bioRxiv [Preprint]. 2024 Sep 24:2024.09.23.614522. doi: 10.1101/2024.09.23.614522. bioRxiv. 2024. Update in: Anal Chem. 2025 Mar 18;97(10):5498-5506. doi: 10.1021/acs.analchem.4c05138. PMID: 39386568 Free PMC article. Updated. Preprint.
-
Polyploid giant cancer cells: origin, possible pathways of formation, characteristics, and mechanisms of regulation.Front Cell Dev Biol. 2024 Jul 11;12:1410637. doi: 10.3389/fcell.2024.1410637. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39055650 Free PMC article. Review.
-
miR-1246 enhances chemo-resistance of polyploid giant cancer cells in H1299 cells by targeting GSK3β/β-catenin.Discov Oncol. 2025 May 24;16(1):901. doi: 10.1007/s12672-025-02756-0. Discov Oncol. 2025. PMID: 40411667 Free PMC article.
-
Polyploid cancer cells reveal signatures of chemotherapy resistance.bioRxiv [Preprint]. 2024 Aug 23:2024.08.19.608632. doi: 10.1101/2024.08.19.608632. bioRxiv. 2024. Update in: Oncogene. 2025 Mar;44(7):439-449. doi: 10.1038/s41388-024-03212-z. PMID: 39229204 Free PMC article. Updated. Preprint.
-
Dormant cancer cells and polyploid giant cancer cells: The roots of cancer recurrence and metastasis.Clin Transl Med. 2024 Feb;14(2):e1567. doi: 10.1002/ctm2.1567. Clin Transl Med. 2024. PMID: 38362620 Free PMC article. Review.
References
-
- Rand CW, Courville CB. Multinucleation of cortical nerve cells at the margins of traumatic lesions of the human brain. J. Neuropathol. Exp. Neurol. 1947;6(1):1–14. - PubMed
-
- Rather LJ. A note on the origin of multinucleated giant cells from vascular channels in tumors; tumors arising in thyroid gland, bone, and soft tissue. AMA Arch Pathol. 1951;52(1):98–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

