Comparison of the efficacy of equivalent doses of dexamethasone, methylprednisolone, and hydrocortisone for treatment of COVID-19-related acute respiratory distress syndrome: a prospective three-arm randomized clinical trial
- PMID: 36624180
- PMCID: PMC9838299
- DOI: 10.1007/s10354-022-00993-4
Comparison of the efficacy of equivalent doses of dexamethasone, methylprednisolone, and hydrocortisone for treatment of COVID-19-related acute respiratory distress syndrome: a prospective three-arm randomized clinical trial
Abstract
Background: This prospective controlled clinical trial aimed to compare the efficacy of methylprednisolone, dexamethasone, and hydrocortisone at equivalent doses in patients with severe COVID-19.
Methods: In total, 106 patients with mild to moderate COVID-19-related acute respiratory distress syndrome (ARDS) were randomized to receive either dexamethasone (6 mg once a day), methylprednisolone (16 mg twice a day), or hydrocortisone (50 mg thrice a day) for up to 10 days. All participants received a standard of care for COVID-19. The primary and secondary efficacy outcomes included all-cause 28-day mortality, clinical status on day 28 assessed using the World Health Organization (WHO) eight-category ordinal clinical scale, number of patients requiring mechanical ventilation and intensive care unit (ICU) care, number of ventilator-free days, length of hospital and ICU stay, change in PaO2:FiO2 ratios during the first 5 days after treatment, and incidence of serious adverse events. P-values below 0.008 based on Bonferroni's multiple-testing correction method were considered statistically significant.
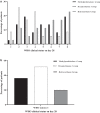
Results: According to the obtained results, there was a trend toward more favorable clinical outcomes in terms of needing mechanical ventilation and ICU care, number of ventilator-free days, change in PaO2:FiO2 ratios during the first 5 days after treatment, clinical status score at day 28, length of ICU and hospital stay, and overall 28-day mortality in patients receiving dexamethasone compared to those receiving methylprednisolone or hydrocortisone; however, likely due to the study's small sample size, the difference between groups reached a significant level only in the case of clinical status score on day 28 (p-value = 0.003). There was no significant difference in the incidence of serious adverse events between the study groups.
Conclusion: Based on the results, severe cases of COVID-19 treated with dexamethasone might have a better clinical status at 28-day follow-up compared to methylprednisolone and hydrocortisone at an equivalent dose. Larger multicenter trials are required to confirm our findings.
Keywords: Corticosteroids; Cytokine storm; Mechanical ventilation; Mortality; SARS-CoV-19 infection; Severe COVID-19 pneumonia.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, ein Teil von Springer Nature.
Conflict of interest statement
A. Taher, M. Lashkari, F. Keramat, S.H. Hashemi, L. Sedighi, J. Poorolajal, and M. Mehrpooya declare that they have no competing interests.
Figures

References
-
- Organization WH . COVID-19 weekly epidemiological update. 58 2021.
-
- Jafari-Oori M, Ghasemifard F, Ebadi A, Karimi L, Rahimi-Bashar F, Jamialahmadi T, Guest PC, Vahedian-Azimi A, Sahebkar A. 18 acute respiratory distress syndrome and COVID-19: a scoping review and meta-analysis. Clin Biol Mol Aspects Covid-19. 2021;1321:211. doi: 10.1007/978-3-030-59261-5_18. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

