Behavioral origin of sound-evoked activity in mouse visual cortex
- PMID: 36624279
- PMCID: PMC9905016
- DOI: 10.1038/s41593-022-01227-x
Behavioral origin of sound-evoked activity in mouse visual cortex
Abstract
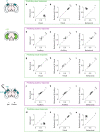
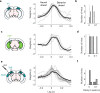
Sensory cortices can be affected by stimuli of multiple modalities and are thus increasingly thought to be multisensory. For instance, primary visual cortex (V1) is influenced not only by images but also by sounds. Here we show that the activity evoked by sounds in V1, measured with Neuropixels probes, is stereotyped across neurons and even across mice. It is independent of projections from auditory cortex and resembles activity evoked in the hippocampal formation, which receives little direct auditory input. Its low-dimensional nature starkly contrasts the high-dimensional code that V1 uses to represent images. Furthermore, this sound-evoked activity can be precisely predicted by small body movements that are elicited by each sound and are stereotyped across trials and mice. Thus, neural activity that is apparently multisensory may simply arise from low-dimensional signals associated with internal state and behavior.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures












References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

