Functional genomics provide key insights to improve the diagnostic yield of hereditary ataxia
- PMID: 36624280
- PMCID: PMC10316781
- DOI: 10.1093/brain/awad009
Functional genomics provide key insights to improve the diagnostic yield of hereditary ataxia
Abstract
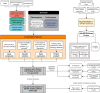
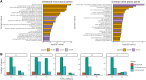
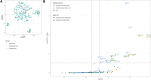
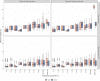
Improvements in functional genomic annotation have led to a critical mass of neurogenetic discoveries. This is exemplified in hereditary ataxia, a heterogeneous group of disorders characterised by incoordination from cerebellar dysfunction. Associated pathogenic variants in more than 300 genes have been described, leading to a detailed genetic classification partitioned by age-of-onset. Despite these advances, up to 75% of patients with ataxia remain molecularly undiagnosed even following whole genome sequencing, as exemplified in the 100 000 Genomes Project. This study aimed to understand whether we can improve our knowledge of the genetic architecture of hereditary ataxia by leveraging functional genomic annotations, and as a result, generate insights and strategies that raise the diagnostic yield. To achieve these aims, we used publicly-available multi-omics data to generate 294 genic features, capturing information relating to a gene's structure, genetic variation, tissue-specific, cell-type-specific and temporal expression, as well as protein products of a gene. We studied these features across genes typically causing childhood-onset, adult-onset or both types of disease first individually, then collectively. This led to the generation of testable hypotheses which we investigated using whole genome sequencing data from up to 2182 individuals presenting with ataxia and 6658 non-neurological probands recruited in the 100 000 Genomes Project. Using this approach, we demonstrated a high short tandem repeat (STR) density within childhood-onset genes suggesting that we may be missing pathogenic repeat expansions within this cohort. This was verified in both childhood- and adult-onset ataxia patients from the 100 000 Genomes Project who were unexpectedly found to have a trend for higher repeat sizes even at naturally-occurring STRs within known ataxia genes, implying a role for STRs in pathogenesis. Using unsupervised analysis, we found significant similarities in genomic annotation across the gene panels, which suggested adult- and childhood-onset patients should be screened using a common diagnostic gene set. We tested this within the 100 000 Genomes Project by assessing the burden of pathogenic variants among childhood-onset genes in adult-onset patients and vice versa. This demonstrated a significantly higher burden of rare, potentially pathogenic variants in conventional childhood-onset genes among individuals with adult-onset ataxia. Our analysis has implications for the current clinical practice in genetic testing for hereditary ataxia. We suggest that the diagnostic rate for hereditary ataxia could be increased by removing the age-of-onset partition, and through a modified screening for repeat expansions in naturally-occurring STRs within known ataxia-associated genes, in effect treating these regions as candidate pathogenic loci.
Keywords: functional genomics; hereditary ataxia; rare disease; repeat expansion disorders; transcriptomics.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests
Figures



References
-
- Boycott KM, Hartley T, Biesecker LG, et al. . A diagnosis for all rare genetic diseases: The horizon and the next frontiers. Cell. 2019;177:32–37. - PubMed
-
- Warman Chardon J, Beaulieu C, Hartley T, Boycott KM, Dyment DA. Axons to exons: The molecular diagnosis of rare neurological diseases by next-generation sequencing. Curr Neurol Neurosci Rep. 2015;15:64. - PubMed

