Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial
- PMID: 36624315
- PMCID: PMC9873554
- DOI: 10.1038/s41591-022-02128-z
Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial
Abstract
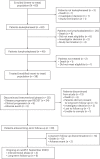
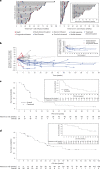
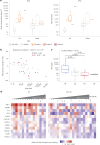
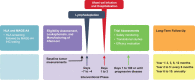
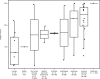
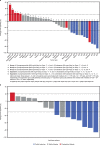
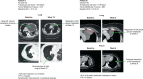
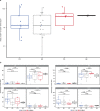
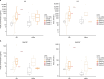
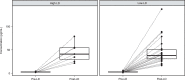
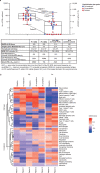
Affinity-optimized T cell receptors can enhance the potency of adoptive T cell therapy. Afamitresgene autoleucel (afami-cel) is a human leukocyte antigen-restricted autologous T cell therapy targeting melanoma-associated antigen A4 (MAGE-A4), a cancer/testis antigen expressed at varying levels in multiple solid tumors. We conducted a multicenter, dose-escalation, phase 1 trial in patients with relapsed/refractory metastatic solid tumors expressing MAGE-A4, including synovial sarcoma (SS), ovarian cancer and head and neck cancer ( NCT03132922 ). The primary endpoint was safety, and the secondary efficacy endpoints included overall response rate (ORR) and duration of response. All patients (N = 38, nine tumor types) experienced Grade ≥3 hematologic toxicities; 55% of patients (90% Grade ≤2) experienced cytokine release syndrome. ORR (all partial response) was 24% (9/38), 7/16 (44%) for SS and 2/22 (9%) for all other cancers. Median duration of response was 25.6 weeks (95% confidence interval (CI): 12.286, not reached) and 28.1 weeks (95% CI: 12.286, not reached) overall and for SS, respectively. Exploratory analyses showed that afami-cel infiltrates tumors, has an interferon-γ-driven mechanism of action and triggers adaptive immune responses. In addition, afami-cel has an acceptable benefit-risk profile, with early and durable responses, especially in patients with metastatic SS. Although the small trial size limits conclusions that can be drawn, the results warrant further testing in larger studies.
© 2023. The Author(s).
Conflict of interest statement
D.S.H. reports grants and personal fees from Adaptimmune related to this research. Outside the submitted work, D.S.H. reports grants from AbbVie, Adlai Nortye, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Fate Therapeutics, Ignyta, Kite, Kyowa, Loxo Oncology, Merck, MedImmune, Mirati, miRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Turning Point Therapeutics, EMD Serono, Erasca, Millenium, Navier, Verstatem and VM Oncology; grants and personal fees from Infinity, Pfizer, Seattle Genetics and Numa; grants, personal fees and non-financial support from Amgen, Genentech, Bayer and Takeda; grants and non-financial support from AstraZeneca, Genmab, GlaxoSmithKline and Eli Lilly; non-financial support from AACR, ASCO, Celgene and Philips; personal fees and non-financial support from Janssen; personal fees from Alpha Insights, Axiom, Baxter, Acuta, GLG, Group H, Guidepoint, HCW Precision, Medscape, Merrimack, Prime Oncology, Trieza Therapeutics, WebMD, ST Cube, Tavistock, Boxer Capital, COG and ECOR1 during the conduct of the study; and roles in Molecular Match (advisor), OncoResponse (founder) and Presagia (advisor). B.V.T. reports personal fees, non-financial support and other from Adaptimmune during the conduct of the study related to this research. Outside the submitted work, B.V.T. reports grants from Pfizer, Merck and Tracon Pharmaceuticals; grants, personal fees, non-financial support and other from GlaxoSmithKline; personal fees from Accuronix Therapeutics, ADRx, Ayala Pharmaceuticals, Cytokinetics, Bayer, Bionest Partners, Intellisphere LLC, Hinshaw & Culbertson LLP, Rodney Law, CRICO Risk Management Foundation and Tracey & Fox Law Firm; having attended advisory board meetings for Apexigen, Daiichi Sankyo, Deciphera Pharmaceuticals, Epizyme, GlaxoSmithKline, Novartis and Eli Lilly; and being a board member for Polaris, outside the submitted work. S.B., C.M., Q.L., A.J.T., J.P.S., R.W., T.T., T.A., S.R., A.S., L.F., J.-M.N., R.B., M.I., R.N., N.B., G.B., Z.W., D.G.B., E.V.W., E.E., P.M.F., E.N. and D.W. report employment by Adaptimmune and stock or stock options in Adaptimmune related to this research. M.L.J. reports grants to the institution from Adaptimmune related to this research. Outside the submitted work, M.L.J. reports grants to the institution from AbbVie, Amgen, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, AstraZeneca, ATRECA, BeiGene, BerGenBio, Birdie Pharmaceuticals/Seven & Eight Biopharmaceuticals, Boehringer Ingelheim, Calithera Biosciences, Checkpoint Theapeutics, Corvus, Cytomx, Daiichi Sankyo, Dracen Pharmaceutics, Dynavax, EMD Serono, Genentech/Roche, Genmab, Genocea, GlaxoSmithKline, Gritstone Oncology, Harpoon Therapeutics, Hengrui Therapeutics, Immunocore, Incyte, Janssen, Eli Lilly, Loxo Oncology, Lycera, Merck, Mirati Therapeutics, Neovia, Novartis, Pfizer, PMV Pharmaceuticals, Regeneron, Ribon Therapeutics, Sanofi, Shattuck Labs, Silicon Therapeutics, Stemcentrx, Syndax, Takeda, Tarveda Therapeutics, TCR2 Therapeutics, TMUNITY Therapeutics, University of Michigan and WindMIL Therapeutics; other from spouse role as a contract lobbyist for Astellas and Otsuka Pharmaceuticals; and other to the institution from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Calithera Biosciences, Celgene, Daiichi Sankyo, Editas Medicine, Eisai, EMD Serono, G1 Therapeutics, Genentech/Roche, GlaxoSmithKline, Gritstone Oncology, Ideaya Biosciences, Incyte, Janssen, Eli Lilly, Loxo Oncology, Merck, Mirati Therapeutics, Novartis, Pfizer, Ribon Therapeutics, Sanofi and WindMIL Therapeutics. A.J.O. reports non-financial support from Adaptimmune during the conduct of the study related to this research. Outside the submitted work, A.J.O. reports personal fees from Merck, Bristol Myers Squibb, Sanofi, Eisai and Pfizer. J.M.C. reports grants from Adaptimmune related to this study. Outside the submitted work, J.M.C. reports grants from Bristol Myers Squibb, CBMG, Spectrum, Medpacto, Bayer, AbbVie, Moderna, GlaxoSmithKline, Array and CGMB; personal fees from Guardant; grants, personal fees and non-financial support from AstraZeneca, Pfizer, NGM Biopharmaceuticals, Merck and Lung Cancer Initiative of North Carolina; and grants and personal fees from Genentech. D.A., P.K., F.D.B., J.K.E., D.K., A.C., A.G.ʼM. and D.A.L. declare no competing interests. G.R.B.J. reports grants or contracts from Adaptimmune related to this study. Outside the submitted work, G.R.B.J. reports grants or contracts from Amgen, Bayer, Elelixis, Daiichi Sankyo, GlaxoSmithKline, Immatics, Immunocore, Incyte, Kite Pharma, Macrogenics, Torque, AstraZeneca, Bristol Myers Squibb, Celgene, Genentech, MedImmune, Merck, Novartis, Roche, Sanofi, Xcovery, Tmunity Therapeutics, Regeneron, BeiGene, Repertoire Immune Medicines, Verastem, CytomX Therapeutics and Duality Biologics; consulting fees from Abbvie, Adicet, Amgen, Ariad, Bayer, Clovis Oncology, AstraZeneca, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Instil Bio, Genentech, Genzyme, Gilead, Eli Lilly, Janssen, MedImmune, Merck, Novartis, Roche, Sanofi, Tyme Oncology, Xcovery, Virogin Biotech, Maverick Therapeutics, BeiGene, Rgeneron, Cytomx Therapeutics, Intervenn Biosciences and Onconova Therapeutics; participation on a Data Safety Monitoring Board or Advisory Board for Virogin Biotech and Maverick Therapeutics; stock or stock options in Virogin Biotech; and other financial or non-financial interests in Johnson & Johnson/Janssen (immediate family member). J.B. works as a contractor for Adaptimmune and contributed to the research reported here. A.G. reports consulting or advisory role for Kite Pharma, Amgen, Atara, Wugen and Celgene; research funding from Kite Pharma, Genentech and Amgen; and honoraria from Kite Pharma, outside the submitted work. Outside the submitted work, P.M. reports personal fees from Bristol Myers Squibb, Celgene, Fate Therapeutics, Janssen, Juno, Karyopharm, Magenta Therapeutics, Oncopeptides and Takeda. M.D. reports personal fees from Adaptimmune related to this research. Outside the submitted work, M.D. reports personal fees from Blueprint and personal fees and non-financial support from Deciphera, Epizyme and Daiichi Sankyo. K.O. reports grants from AstraZeneca and Tessaro outside the submitted work and patent PCT/US2014025673, licensed to Tactiva Therapeutics, and patent PCT/US2014025456, licensed to Tactiva Therapeutics. M.O.B. reports a consulting or advisory role with Adaptimmune related to this research. Outside the submitted work, M.OB. reports honoraria from Bristol Myers Squibb, Merck, Novartis and Roche; a consulting or advisory role with Bristol Myers Squibb, EMD Serono, Genzyme, GlaxoSmithKline, Immunocore, Immunovaccine, Instil Bio, Iovance Biotherapeutics, LaRoche Posay, Merck, Novartis, Sanofi and Sun Pharma; research funding from Merck and Takara; and expert testimony for Merck.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

