Apicobasal transferrin receptor localization and trafficking in brain capillary endothelial cells
- PMID: 36624498
- PMCID: PMC9830855
- DOI: 10.1186/s12987-022-00404-1
Apicobasal transferrin receptor localization and trafficking in brain capillary endothelial cells
Erratum in
-
Correction to: Apicobasal transferrin receptor localization and trafficking in brain capillary endothelial cells.Fluids Barriers CNS. 2023 Jun 19;20(1):48. doi: 10.1186/s12987-023-00452-1. Fluids Barriers CNS. 2023. PMID: 37337211 Free PMC article. No abstract available.
Abstract
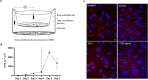
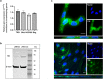
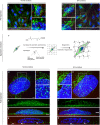
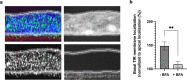
The detailed mechanisms by which the transferrin receptor (TfR) and associated ligands traffic across brain capillary endothelial cells (BECs) of the CNS-protective blood-brain barrier constitute an important knowledge gap within maintenance and regulation of brain iron homeostasis. This knowledge gap also presents a major obstacle in research aiming to develop strategies for efficient receptor-mediated drug delivery to the brain. While TfR-mediated trafficking from blood to brain have been widely studied, investigation of TfR-mediated trafficking from brain to blood has been limited. In this study we investigated TfR distribution on the apical and basal plasma membranes of BECs using expansion microscopy, enabling sufficient resolution to separate the cellular plasma membranes of these morphological flat cells, and verifying both apical and basal TfR membrane domain localization. Using immunofluorescence-based transcellular transport studies, we delineated endosomal sorting of TfR endocytosed from the apical and basal membrane, respectively, as well as bi-directional TfR transcellular transport capability. The findings indicate different intracellular sorting mechanisms of TfR, depending on the apicobasal trafficking direction across the BBB, with the highest transcytosis capacity in the brain-to-blood direction. These results are of high importance for the current understanding of brain iron homeostasis. Also, the high level of TfR trafficking from the basal to apical membrane of BECs potentially explains the low transcytosis which are observed for the TfR-targeted therapeutics to the brain parenchyma.
Keywords: Apicobasal polarity; Blood–brain barrier; Brain drug delivery; Brain endothelial cells; Expansion microscopy; Intracellular trafficking; Transferrin receptor (TfR).
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

