6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway
- PMID: 36624878
- PMCID: PMC9825225
- DOI: 10.1155/2022/3027514
6-Gingerol Alleviates Ferroptosis and Inflammation of Diabetic Cardiomyopathy via the Nrf2/HO-1 Pathway
Abstract
Background: Diabetes mellitus (DM) can induce cardiomyocyte injury and lead to diabetic cardiomyopathy (DCM) which presently has no specific treatments and consequently increase risk of mortality.
Objective: To characterize the therapeutic effect of 6-gingerol (6-G) on DCM and identify its potential mechanism.
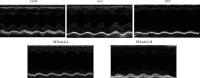
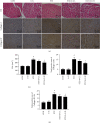
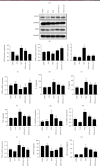
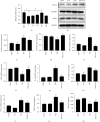
Methods: In vivo streptozotocin- (STZ-) induced DM model was established by using a high-fat diet and STZ, followed by low-dose (25 mg/kg) and high-dose (75 mg/kg) 6-G intervention. For an in vitro DCM model, H9c2 rat cardiomyoblast cells were stimulated with high glucose (glucose = 33 mM) and palmitic acid (100 μM) and then treated with 6-G (100 μM). Histological and echocardiographic analyses were used to assess the effect of 6-G on cardiac structure and function in DCM. Western blotting, ELISA, and real-time qPCR were used to assess the expression of ferroptosis, inflammation, and the Nrf2/HO-1 pathway-related proteins and RNAs. Protein expression of collagen I and collagen III was assessed by immunohistochemistry, and kits were used to assay SOD, MDA, and iron levels.
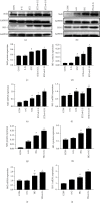
Results: The results showed that 6-G decreased cardiac injury in both mouse and cell models of DCM. The cardiomyocyte hypertrophy and interstitial fibrosis were attenuated by 6-G treatment in vivo and resulted in an improved heart function. 6-G inhibited the expression of ferroptosis-related protein FACL4 and the content of iron and enhanced the expression of anti-ferroptosis-related protein GPX4. In addition, 6-G also diminished the secretion of inflammatory cytokines, including IL-1β, IL-6, and TNF-α. 6-G treatment activated the Nrf2/HO-1 pathway, enhanced antioxidative stress capacity proved by increased activity of SOD, and decreased MDA production. Compared with in vivo, 6-G treatment of H9c2 cells treated with high glucose and palmitic acid could produce a similar effect.
Conclusion: These findings suggest that 6-G could protect against DCM by the mechanism of ferroptosis inhibition and inflammation reduction via enhancing the Nrf2/HO-1 pathway.
Copyright © 2022 Shenglin Wu et al.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures
References
-
- Yap J., Tay W. T., Teng T. K., et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. Journal of the American Heart Association . 2019;8(17, article e013114) doi: 10.1161/JAHA.119.013114. - DOI - PMC - PubMed
-
- Tate M., Grieve D. J., Ritchie R. H. Are Targeted Therapies for Diabetic Cardiomyopathy on the Horizon? Clinical Science. Clinical Science . 2017;131:897–915. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

