Extracorporeal life support provision in COVID-19 patients - An international EuroELSO 2022 update survey
- PMID: 36625181
- PMCID: PMC9834627
- DOI: 10.1177/02676591221151034
Extracorporeal life support provision in COVID-19 patients - An international EuroELSO 2022 update survey
Abstract
Introduction: An analysis on the ECLS use for patients with respiratory or cardiac support in COVID-19 based on an international response to EuroELSO survey, aims to generate a more comprehensive understanding of ECLS role during the recent viral pandemic.
Methods: EuroELSO announced the survey at the 10th annual congress in London, May 2022. The survey covered 26 multiple-choice questions.
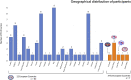
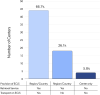
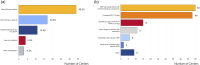
Results: The survey returned 69 questionnaires from 62 centers across 22 European countries and seven centers across five non-European countries. Most of the centers providing ECLS for COVID-19 patients had more than 30 runs for respiratory support since December 2019. In the same period, at least 31 runs in adult COVID-19 patients have been performed in 48 of 69 centers (69.6%). The reported pediatric data from 18 centers is limited to less than the patients per center.
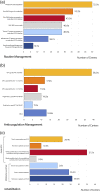
Conclusion: Majority of the COVID-19 patients received respiratory ECLS support and adult patients dominated. The indications and contraindications are broadly aligned with available guidelines. Most of the centers considered age >65 or biological age as a relative or absolute contraindication for ECLS in COVID-19. ECLS withdrawal criteria in COVID-19 are controversial because the long-term outcomes after ECLS in COVID-19 and the impact of critical illness and the impact of long-COVID are still not known.
Keywords: COVID-19; ECLS; ECMO; SARS-CoV-2; extracorporeal life support; extracorporeal membrane oxygenation.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Prof. Dr Lorusso is a consultant for Medtronic, Getinge, Abiomed, and LivaNova and medical advisory board member for Xenios and EUROSETS, all unrelated to this work, all honoraria to the university for research funding. Dr Di Nardo is medical advisory board member for EUROSETS. Dr Mirko Belliato medical advisory board member for EUROSETS srl (Italy) and congress speaker for ESTOR spa (Italy) and Hamilton Medical (Swiss). Dr Lars Mikael Broman is a medical advisory board member of Eurosets Srl (Italy), Xenios AG, (Ger), and HemoCue AB (Swe).
Figures
Similar articles
-
Extracorporeal life support in COVID-19-related acute respiratory distress syndrome: A EuroELSO international survey.Artif Organs. 2021 May;45(5):495-505. doi: 10.1111/aor.13940. Epub 2021 Mar 28. Artif Organs. 2021. PMID: 33590542 Free PMC article.
-
"Bridging the Gap" international ECLS training and simulation - evaluation of the 10th educational corner on EuroELSO congress 2022 in London, United Kingdom.Perfusion. 2023 May;38(1_suppl):3-12. doi: 10.1177/02676591231157273. Epub 2023 Apr 20. Perfusion. 2023. PMID: 37078917
-
GEospatial aNalysis of ExtRacorporeal membrane oxygenATion in Europe (GENERATE).Perfusion. 2023 May;38(1_suppl):24-39. doi: 10.1177/02676591231160471. Epub 2023 Mar 6. Perfusion. 2023. PMID: 36879353
-
COVID-19 and Extracorporeal Membrane Oxygenation.Adv Exp Med Biol. 2021;1353:173-195. doi: 10.1007/978-3-030-85113-2_10. Adv Exp Med Biol. 2021. PMID: 35137374 Review.
-
Extracorporeal Life Support (ECLS): A Review and Focus on Considerations for COVID-19.Shock. 2021 Jun 1;55(6):742-751. doi: 10.1097/SHK.0000000000001677. Shock. 2021. PMID: 33393754 Review.
References
-
- Martucci G, Słomka A, Lebowitz SE, et al.COVID-19 and Extracorporeal Membrane Oxygenation. Adv Exp Med Biol 2021; 1353: 173–195. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

