Preliminary evidence supporting a new enzymatic debridement product for use in chronic wounds
- PMID: 36625224
- PMCID: PMC10333010
- DOI: 10.1111/iwj.14079
Preliminary evidence supporting a new enzymatic debridement product for use in chronic wounds
Abstract
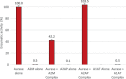
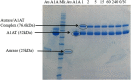
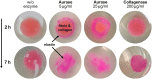
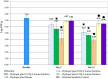
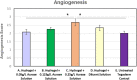
A new recombinant proteolytic enzyme, isolated from maggot saliva, with fibrinolytic action has been investigated through a series of non-clinical toxicology and in-vitro/in-vivo pharmacology studies to explore its potential safety and efficacy as an enzymatic debridement agent for use in chronic wounds. Studies indicate that the enzyme has a good safety profile. When locally administered, it is not detrimental to wound healing, is non-sensitising and is rapidly inactivated in the systemic circulation. Adverse effects are limited, at very high concentrations, to transient erythema at the site of application. In-vitro testing indicates that the enzyme, whilst selective for fibrin, has additional proteolytic action against collagen and elastin, with enzymatic action for all three substrates being dose dependent. In-vivo, we used an established MRSA biofilm model, in which microbiological counts were used as a surrogate for debridement efficacy. Here, we showed that higher concentrations of the enzyme in a formulated proprietary gel, significantly reduced MRSA counts over a period of 2 to 14 days, and significantly improved the vascularity of the wound at 14 days. Together, these data support the potential for this maggot-derived proteolytic enzyme as a clinically effective debriding agent.
Keywords: chronic; debridement; pharmacology; toxicology; wounds.
© 2022 SolasCure Ltd. International Wound Journal published by Medicalhelplines.com Inc (3M) and John Wiley & Sons Ltd.
Conflict of interest statement
All authors are associated with SolasCure Ltd either as employees, shareholders and/or company advisers.
Figures

References
-
- Naik G, Harding K. Maggot debridement therapy: the current perspectives. Chronic Wound Care Manag Res. 2017;4:121‐128.
-
- Naafs MA. The Maggots—Professional Wound Cleaners: Global Biofilm Advisory Council; 2017; https://infectioncontrol.tips/2017/10/31/maggots-professional-wound-clea....
-
- Grassberger M, Fleischmann W. The biobag ‐ a new device for the application of medicinal maggots. Dermatology. 2002;204(4):306. - PubMed
-
- Preuss SF, Stenzel MJ, Esriti A. The successful use of maggots in necrotizing fasciitis of the neck: a case report. Head Neck. 2004;26(8):747‐750. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

