Mucociliary transport deficiency and disease progression in Syrian hamsters with SARS-CoV-2 infection
- PMID: 36625345
- PMCID: PMC9870055
- DOI: 10.1172/jci.insight.163962
Mucociliary transport deficiency and disease progression in Syrian hamsters with SARS-CoV-2 infection
Abstract
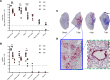
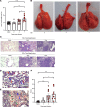
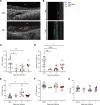
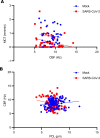
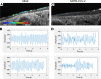
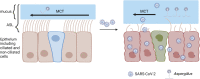
Substantial clinical evidence supports the notion that ciliary function in the airways is important in COVID-19 pathogenesis. Although ciliary damage has been observed in both in vitro and in vivo models, the extent or nature of impairment of mucociliary transport (MCT) in in vivo models remains unknown. We hypothesize that SARS-CoV-2 infection results in MCT deficiency in the airways of golden Syrian hamsters that precedes pathological injury in lung parenchyma. Micro-optical coherence tomography was used to quantitate functional changes in the MCT apparatus. Both genomic and subgenomic viral RNA pathological and physiological changes were monitored in parallel. We show that SARS-CoV-2 infection caused a 67% decrease in MCT rate as early as 2 days postinfection (dpi) in hamsters, principally due to 79% diminished airway coverage of motile cilia. Correlating quantitation of physiological, virological, and pathological changes reveals steadily descending infection from the upper airways to lower airways to lung parenchyma within 7 dpi. Our results indicate that functional deficits of the MCT apparatus are a key aspect of COVID-19 pathogenesis, may extend viral retention, and could pose a risk factor for secondary infection. Clinically, monitoring abnormal ciliated cell function may indicate disease progression. Therapies directed toward the MCT apparatus deserve further investigation.
Keywords: COVID-19; Respiration.
Conflict of interest statement
Figures

Update of
-
Mucociliary Transport Deficiency and Disease Progression in Syrian Hamsters with SARS-CoV-2 Infection.bioRxiv [Preprint]. 2022 Jan 18:2022.01.16.476016. doi: 10.1101/2022.01.16.476016. bioRxiv. 2022. Update in: JCI Insight. 2023 Jan 10;8(1):e163962. doi: 10.1172/jci.insight.163962. PMID: 35075457 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

