Rapid desensitization through immunoadsorption during cardiopulmonary bypass. A novel method to facilitate human leukocyte antigen incompatible heart transplantation
- PMID: 36625378
- PMCID: PMC10943618
- DOI: 10.1177/02676591221151035
Rapid desensitization through immunoadsorption during cardiopulmonary bypass. A novel method to facilitate human leukocyte antigen incompatible heart transplantation
Abstract
Background: Anti-human leukocyte antigen (HLA)-antibody production represents a major barrier to heart transplantation, limiting recipient compatibility with potential donors and increasing the risk of complications with poor waiting-list outcomes. Currently there is no consensus to when desensitization should take place, and through what mechanism, meaning that sensitized patients must wait for a compatible donor for many months, if not years. We aimed to determine if intraoperative immunoadsorption could provide a potential desensitization methodology.
Methods: Anti-HLA antibody-containing whole blood was added to a Cardiopulmonary bypass (CPB) circuit set up to mimic a 20 kg patient undergoing heart transplantation. Plasma was separated and diverted to a standalone, secondary immunoadsorption system, with antibody-depleted plasma returned to the CPB circuit. Samples for anti-HLA antibody definition were taken at baseline, when combined with the CPB prime (on bypass), and then every 20 min for the duration of treatment (total 180 min).
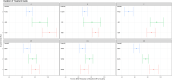
Results: A reduction in individual allele median fluorescence intensity (MFI) to below clinically relevant levels (<1000 MFI), and in the majority of cases below the lower positive detection limit (<500 MFI), even in alleles with a baseline MFI >4000 was demonstrated. Reduction occurred in all cases within 120 min, demonstrating efficacy in a time period usual for heart transplantation. Flowcytometric crossmatching of suitable pseudo-donor lymphocytes demonstrated a change from T cell and B cell positive channel shifts to negative, demonstrating a reduction in binding capacity.
Conclusions: Intraoperative immunoadsorption in an ex vivo setting demonstrates clinically relevant reductions in anti-HLA antibodies within the normal timeframe for heart transplantation. This method represents a potential desensitization technique that could enable sensitized children to accept a donor organ earlier, even in the presence of donor-specific anti-HLA antibodies.
Keywords: cardiopulmonary bypass; human leukocyte antigen; immunoadsorption; paediatric; transplantation.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. New Engl J Med 1969; 280(14): 735–739. - PubMed
-
- Nwakanma LU, Williams JA, Weiss ES, et al. Influence of pretransplant panel-reactive antibody on outcomes in 8, 160 heart transplant recipients in recent era. Ann Thorac Surg 2007; 84(5): 1556–1562. ; discussion 62-3. - PubMed
-
- John R, Lietz K, Schuster M, et al. Immunologic sensitization in recipients of left ventricular assist devices. J Thorac Cardiovasc Surg 2003; 125(3): 578–591. - PubMed
-
- Al-Mohaissen MA, Virani SA. Allosensitization in heart transplantation: an overview. Can J Cardiol 2014; 30(2): 161–172. - PubMed
-
- Colvin MM, Cook JL, Chang PP, et al. Sensitization in heart transplantation: emerging knowledge: a scientific statement from the American heart association. Circulation 2019; 139(12): e53–e78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

