Quality-of-life outcomes and risk prediction for patients randomized to nivolumab plus ipilimumab vs nivolumab on LungMAP-S1400I
- PMID: 36625510
- PMCID: PMC10086628
- DOI: 10.1093/jnci/djad003
Quality-of-life outcomes and risk prediction for patients randomized to nivolumab plus ipilimumab vs nivolumab on LungMAP-S1400I
Abstract
Background: An important issue for patients with cancer treated with novel therapeutics is how they weigh the effects of treatment on survival and quality of life (QOL). We compared QOL in patients enrolled to SWOG S1400I, a substudy of the LungMAP biomarker-driven master protocol.
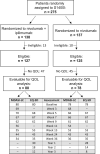
Methods: SWOG S1400I was a randomized phase III trial comparing nivolumab plus ipilimumab vs nivolumab for treatment of immunotherapy-naïve disease in advanced squamous cell lung cancer. The primary endpoint was the MD Anderson Symptom Inventory-Lung Cancer severity score at week 7 and week 13 with a target difference of 1.0 points, assessed using multivariable linear regression. A composite risk model for progression-free and overall survival was derived using best-subset selection.
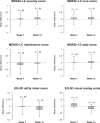
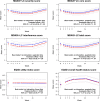
Results: Among 158 evaluable patients, median age was 67.6 years and most were male (66.5%). The adjusted MD Anderson Symptom Inventory-Lung Cancer severity score was 0.04 points (95% confidence interval [CI] = -0.44 to 0.51 points; P = .89) at week 7 and 0.12 points (95% CI = -0.41 to 0.65; P = .66) at week 13. A composite risk model showed that patients with high levels of appetite loss and shortness of breath had a threefold increased risk of progression or death (hazard ratio [HR] = 3.06, 95% CI = 1.88 to 4.98; P < .001) and that those with high levels of both appetite loss and work limitations had a fivefold increased risk of death (HR = 5.60, 95% CI = 3.27 to 9.57; P < .001)-compared with those with neither risk category.
Conclusions: We found no evidence of a benefit of ipilimumab added to nivolumab compared with nivolumab alone for QOL in S1400I. A risk model identified patients at high risk of poor survival, demonstrating the prognostic relevance of baseline patient-reported outcomes even in those with previously treated advanced cancer.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
The importance of patient-reported outcomes in pragmatic clinical trials.J Natl Cancer Inst. 2023 Apr 11;115(4):352-354. doi: 10.1093/jnci/djad037. J Natl Cancer Inst. 2023. PMID: 36805254 Free PMC article. No abstract available.
Similar articles
-
Nivolumab Plus Ipilimumab vs Nivolumab for Previously Treated Patients With Stage IV Squamous Cell Lung Cancer: The Lung-MAP S1400I Phase 3 Randomized Clinical Trial.JAMA Oncol. 2021 Sep 1;7(9):1368-1377. doi: 10.1001/jamaoncol.2021.2209. JAMA Oncol. 2021. PMID: 34264316 Free PMC article. Clinical Trial.
-
First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in metastatic non-small cell lung cancer: CheckMate 9LA 2-year patient-reported outcomes.Eur J Cancer. 2023 Apr;183:174-187. doi: 10.1016/j.ejca.2023.01.015. Epub 2023 Jan 28. Eur J Cancer. 2023. PMID: 36871487
-
Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma.N Engl J Med. 2022 Feb 3;386(5):449-462. doi: 10.1056/NEJMoa2111380. N Engl J Med. 2022. PMID: 35108470 Clinical Trial.
-
Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden.N Engl J Med. 2018 May 31;378(22):2093-2104. doi: 10.1056/NEJMoa1801946. Epub 2018 Apr 16. N Engl J Med. 2018. PMID: 29658845 Free PMC article. Clinical Trial.
-
Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer.N Engl J Med. 2019 Nov 21;381(21):2020-2031. doi: 10.1056/NEJMoa1910231. Epub 2019 Sep 28. N Engl J Med. 2019. PMID: 31562796 Clinical Trial.
Cited by
-
The importance of patient-reported outcomes in pragmatic clinical trials.J Natl Cancer Inst. 2023 Apr 11;115(4):352-354. doi: 10.1093/jnci/djad037. J Natl Cancer Inst. 2023. PMID: 36805254 Free PMC article. No abstract available.
-
Prognostic models for immunotherapy in non-small cell lung cancer: A comprehensive review.Heliyon. 2024 Apr 17;10(8):e29840. doi: 10.1016/j.heliyon.2024.e29840. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681577 Free PMC article. Review.
-
Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage.Pharmaceutics. 2024 May 29;16(6):732. doi: 10.3390/pharmaceutics16060732. Pharmaceutics. 2024. PMID: 38931856 Free PMC article. Review.
-
Exploring the role of health-related quality of life measures in predictive modelling for oncology: a systematic review.Qual Life Res. 2025 Feb;34(2):305-323. doi: 10.1007/s11136-024-03820-y. Epub 2024 Dec 9. Qual Life Res. 2025. PMID: 39652111 Free PMC article.
-
Novel Approach to Accelerate Lung Cancer Research: Lung-MAP and the Potential of Public-Private Partnerships.Clin Cancer Res. 2024 Jan 5;30(1):29-32. doi: 10.1158/1078-0432.CCR-23-2690. Clin Cancer Res. 2024. PMID: 37903180 Free PMC article.
References
-
- Iyer S, Roughley A, Rider A, et al.The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22(1):181-187. - PubMed
-
- Gridelli C, Ardizzoni A, Le Chevalier T, et al.Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Ann Oncol. 2004;15(3):419-426. - PubMed
-
- Eton DT, Fairclough DL, Cella D, et al.Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21(8):1536-1543. - PubMed
-
- Gotay CC, Kawamoto CT, Bottomley A, et al.The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355-1363. - PubMed

