Membranized Coacervate Microdroplets: from Versatile Protocell Models to Cytomimetic Materials
- PMID: 36625520
- PMCID: PMC9910039
- DOI: 10.1021/acs.accounts.2c00696
Membranized Coacervate Microdroplets: from Versatile Protocell Models to Cytomimetic Materials
Abstract
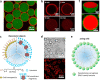
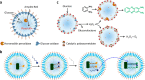
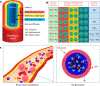
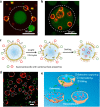
Although complex coacervate microdroplets derived from associative phase separation of counter-charged electrolytes have emerged as a broad platform for the bottom-up construction of membraneless, molecularly crowded protocells, the absence of an enclosing membrane limits the construction of more sophisticated artificial cells and their use as functional cytomimetic materials. To address this problem, we and others have recently developed chemical-based strategies for the membranization of preformed coacervate microdroplets. In this Account, we review our recent work on diverse coacervate systems using a range of membrane building blocks and assembly processes. First, we briefly introduce the unusual nature of the coacervate/water interface, emphasizing the ultralow interfacial tension and broad interfacial width as physiochemical properties that require special attention in the judicious design of membranized coacervate microdroplets. Second, we classify membrane assembly into two different approaches: (i) interfacial self-assembly by using diverse surface-active building blocks such as molecular amphiphiles (fatty acids, phospholipids, block copolymers, protein-polymer conjugates) or nano- and microscale objects (liposomes, nanoparticle surfactants, cell fragments, living cells) with appropriate wettability; and (ii) coacervate droplet-to-vesicle reconfiguration by employing auxiliary surface reconstruction agents or triggering endogenous transitions (self-membranization) under nonstoichiometric (charge mismatched) conditions. We then discuss the key cytomimetic behaviors of membranized coacervate-based model protocells. Customizable permeability is achieved by synergistic effects operating between the molecularly crowded coacervate interior and surrounding membrane. In contrast, metabolic-like endogenous reactivity, diffusive chemical signaling, and collective chemical operations occur specifically in protocell networks comprising diverse populations of membranized coacervate microdroplets. In each case, these cytomimetic behaviors can give rise to functional microscale materials capable of promising cell-like applications. For example, immobilizing spatially segregated enzyme-loaded phospholipid-coated coacervate protocells in concentrically tubular hydrogels delivers prototissue-like bulk materials that generate nitric oxide in vitro, enabling platelet deactivation and inhibition of blood clot formation. Alternatively, therapeutic protocells with in vivo vasoactivity, high hemocompatibility, and increased blood circulation times are constructed by spontaneous assembly of hemoglobin-containing cell-membrane fragments on the surface of enzyme-loaded coacervate microdroplets. Higher-order properties such as artificial endocytosis are achieved by using nanoparticle-caged coacervate protocell hosts that selectively and actively capture guest nano- and microscale objects by responses to exogenous stimuli or via endogenous enzyme-mediated reactions. Finally, we discuss the current limitations in the design and programming of membranized coacervate microdroplets, which may help to guide future directions in this emerging research area. Taken together, we hope that this Account will inspire new advances in membranized coacervate microdroplets and promote their application in the development of integrated protocell models and functional cytomimetic materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

